Page 178 - Handbook of Plastics Technologies
P. 178
THERMOSETS
3.48 CHAPTER 3
TABLE 3.45 Thermoplastic Polyimide Temperature Limits
PEI PAI TMPI fpi
T , °C 340
g
HDT, °C 207–221 278–282 232–257
Continuous service, °C 170–180 371
Embrittlement, hr/200°C >2000
250°C 250
T , °C 450–510
d
and 250 hr at 250°C, and decomposition temperatures of 450 to 510°C. And fluorinated
polyimides containing the hexafluoroisopropylidene group have been reported with tem-
peratures like T = 340°C and continuous service temperature 371°C.
g
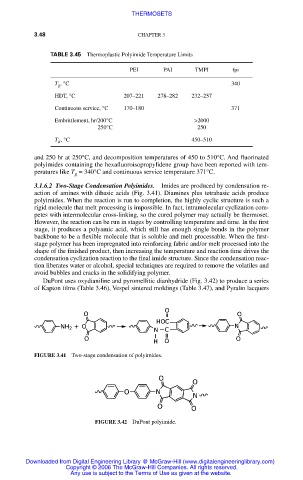
3.1.6.2 Two-Stage Condensation Polyimides. Imides are produced by condensation re-
action of amines with dibasic acids (Fig. 3.41). Diamines plus tetrabasic acids produce
polyimides. When the reaction is run to completion, the highly cyclic structure is such a
rigid molecule that melt processing is impossible. In fact, intramolecular cyclization com-
petes with intermolecular cross-linking, so the cured polymer may actually be thermoset.
However, the reaction can be run in stages by controlling temperature and time. In the first
stage, it produces a polyamic acid, which still has enough single bonds in the polymer
backbone to be a flexible molecule that is soluble and melt processable. When the first-
stage polymer has been impregnated into reinforcing fabric and/or melt processed into the
shape of the finished product, then increasing the temperature and reaction time drives the
condensation cyclization reaction to the final imide structure. Since the condensation reac-
tion liberates water or alcohol, special techniques are required to remove the volatiles and
avoid bubbles and cracks in the solidifying polymer.
DuPont uses oxydianiline and pyromellitic dianhydride (Fig. 3.42) to produce a series
of Kapton films (Table 3.46), Vespel sintered moldings (Table 3.47), and Pyralin lacquers
FIGURE 3.41 Two-stage condensation of polyimides.
FIGURE 3.42 DuPont polyimide.
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2006 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.