Page 85 - Handbook of Surface Improvement and Modification
P. 85
80 Tackifiers
pinene and ββ-pinene or limonene (natural rubber, or polyisoprene, is a polyterpene which
5
consists of up to 1,500-15,000 isoprene units.). Commercial terpene phenol resins are
5
produced by reacting a terpene with a phenol in the presence of a catalyst.
The near infrared absorbing abietic acid derivative was used in the hot-melt adhesive
6
as a NIR sensitive trigger. The esterification posed some difficulties because of a hin-
6
dered nature of carboxyl group in abietic acid.
Hydrocarbon resins are non-polar, and thus highly compatible with non-polar rub-
bers and polymers but poorly compatible with the polar systems, such as acrylic copoly-
7
mers, polyurethanes, and polyamides. Diels-Alder reaction of dicyclopentadiene
monomer and sorbic acid was used to graft sorbic acid on dicyclopentadiene tackifier in
7
the acrylic adhesive (Figure 6.8). The increased polar content of tackifier contributed to
7
the increase of peel adhesion to stainless steel panels. Grafting of sorbic acid caused an
o
increase in softening point of tackifier (sorbic acid content in %/softening point in C=0/
7
122, 10/128, 20/132, 30/140, 40/142).
The tack performance of tackified pressure-sensitive adhesives was affected by the
glass transition temperature of bulk pressure-sensitive adhesives, as well as by the misci-
8
bility and tackifier contents. The softening point of water-based acrylic pressure-sensitive
adhesive was changing with type of tackifier (no tackifier − 58, glycerol ester of stabilized
rosin − 100, pentaerythritol ester of stabilized rosin − 125, and pentaerythritol ester of
o
polymerized rosin − 160 C) as well as its glass transition temperature (no tackifier − 49,
glycerol ester of stabilized rosin − 55, pentaerythritol ester of stabilized rosin − 78, and
o
8
pentaerythritol ester of polymerized rosin − 103 C). The first of these systems was misci-
8
ble and the remaining two immiscible (Figure 6.9). In the miscible blends, the probe tack
strength of adhesive was increased with increasing tackifier concentration unlike in
immiscible adhesive blends where the maximum probe tack strength was shifted toward a
lower debonding rate as the tacki-
8
fier content was increased.
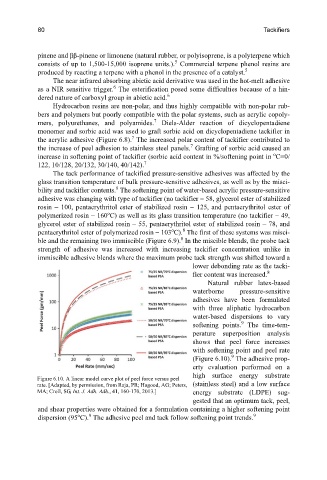
Natural rubber latex-based
waterborne pressure-sensitive
adhesives have been formulated
with three aliphatic hydrocarbon
water-based dispersions to vary
9
softening points. The time-tem-
perature superposition analysis
shows that peel force increases
with softening point and peel rate
9
(Figure 6.10). The adhesive prop-
erty evaluation performed on a
high surface energy substrate
Figure 6.10. A linear model curve plot of peel force versus peel
rate. [Adapted, by permission, from Raja, PR; Hagood, AG; Peters, (stainless steel) and a low surface
MA; Croll, SG, Int. J. Adh. Adh., 41, 160-170, 2013.] energy substrate (LDPE) sug-
gested that an optimum tack, peel,
and shear properties were obtained for a formulation containing a higher softening point
9
o
9
dispersion (95 C). The adhesive peel and tack follow softening point trends.