Page 90 - Illustrated Pocket Dictionary of Chromatography
P. 90
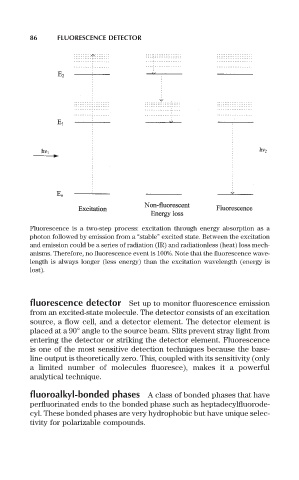
86 FLUORESCENCE DETECTOR
Fluorescence is a two-step process: excitation through energy absorption as a
photon followed by emission from a “stable” excited state. Between the excitation
and emission could be a series of radiation (IR) and radiationless (heat) loss mech-
anisms. Therefore, no fluorescence event is 100%. Note that the fluorescence wave-
length is always longer (less energy) than the excitation wavelength (energy is
lost).
fluorescence detector Set up to monitor fluorescence emission
from an excited-state molecule. The detector consists of an excitation
source, a flow cell, and a detector element. The detector element is
placed at a 90° angle to the source beam. Slits prevent stray light from
entering the detector or striking the detector element. Fluorescence
is one of the most sensitive detection techniques because the base-
line output is theoretically zero. This, coupled with its sensitivity (only
a limited number of molecules fluoresce), makes it a powerful
analytical technique.
fluoroalkyl-bonded phases A class of bonded phases that have
perfluorinated ends to the bonded phase such as heptadecylfluorode-
cyl. These bonded phases are very hydrophobic but have unique selec-
tivity for polarizable compounds.