Page 373 - Industrial Wastewater Treatment, Recycling and Reuse
P. 373
Phenolic Wastewater Treatment: Development and Applications of New Adsorbent Materials 345
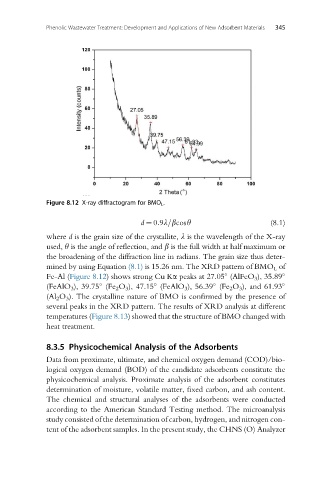
Figure 8.12 X-ray diffractogram for BMO L .
d ¼ 0:9l=bcosy (8.1)
where d is the grain size of the crystallite, l is the wavelength of the X-ray
used, y is the angle of reflection, and b is the full width at half maximum or
the broadening of the diffraction line in radians. The grain size thus deter-
mined by using Equation (8.1) is 15.26 nm. The XRD pattern of BMO L of
Fe-Al (Figure 8.12) shows strong Cu Ka peaks at 27.05 (AlFeO 3 ), 35.89
(FeAlO 3 ), 39.75 (Fe 2 O 3 ), 47.15 (FeAlO 3 ), 56.39 (Fe 2 O 3 ), and 61.93
(Al 2 O 3 ). The crystalline nature of BMO is confirmed by the presence of
several peaks in the XRD pattern. The results of XRD analysis at different
temperatures (Figure 8.13) showed that the structure of BMO changed with
heat treatment.
8.3.5 Physicochemical Analysis of the Adsorbents
Data from proximate, ultimate, and chemical oxygen demand (COD)/bio-
logical oxygen demand (BOD) of the candidate adsorbents constitute the
physicochemical analysis. Proximate analysis of the adsorbent constitutes
determination of moisture, volatile matter, fixed carbon, and ash content.
The chemical and structural analyses of the adsorbents were conducted
according to the American Standard Testing method. The microanalysis
study consisted of the determination of carbon, hydrogen, and nitrogen con-
tent of the adsorbent samples. In the present study, the CHNS (O) Analyzer