Page 372 - Industrial Wastewater Treatment, Recycling and Reuse
P. 372
344 Industrial Wastewater Treatment, Recycling, and Reuse
0.0035 0.007
0.0030 0.005
Pore volume (cm 3 /g A°) 0.0020 Pore Volume (cm 3 /g A°) 0.006
0.0025
0.004
0.003
0.0015
0.0010
0.0005 0.002
0.001
0.0000 0.000
0 500 1000 1500 2000 0 200 400 600 800 1000 1200
(a) Pore diameter (A°) (b) Pore diameter (A°)
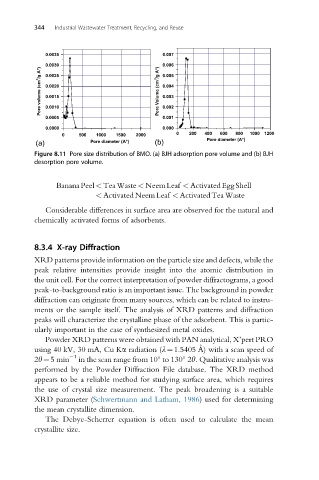
Figure 8.11 Pore size distribution of BMO. (a) BJH adsorption pore volume and (b) BJH
desorption pore volume.
Banana Peel < Tea Waste < NeemLeaf < ActivatedEgg Shell
< ActivatedNeem Leaf < ActivatedTea Waste
Considerable differences in surface area are observed for the natural and
chemically activated forms of adsorbents.
8.3.4 X-ray Diffraction
XRD patterns provide information on the particle size and defects, while the
peak relative intensities provide insight into the atomic distribution in
the unit cell. For the correct interpretation of powder diffractograms, a good
peak-to-background ratio is an important issue. The background in powder
diffraction can originate from many sources, which can be related to instru-
ments or the sample itself. The analysis of XRD patterns and diffraction
peaks will characterize the crystalline phase of the adsorbent. This is partic-
ularly important in the case of synthesized metal oxides.
Powder XRD patterns were obtained with PAN analytical, X’pert PRO
˚
using 40 kV, 30 mA, Cu Ka radiation (l¼1.5405 A) with a scan speed of
2y¼5 min 1 in the scan range from 10 to 130 2y. Qualitative analysis was
performed by the Powder Diffraction File database. The XRD method
appears to be a reliable method for studying surface area, which requires
the use of crystal size measurement. The peak broadening is a suitable
XRD parameter (Schwertmann and Latham, 1986) used for determining
the mean crystallite dimension.
The Debye-Scherrer equation is often used to calculate the mean
crystallite size.