Page 135 - Instrumentation Reference Book 3E
P. 135
120 Measurement of density
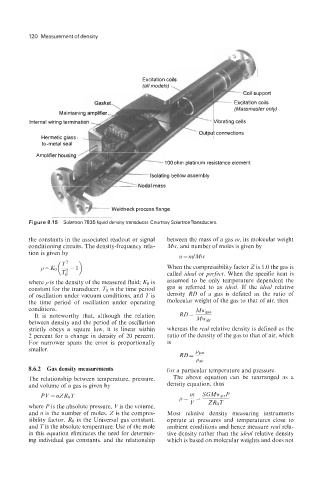
Excitation c
(all models)
A
G E?-' - Excitation coils
(Massmaster only)
Maintaining amp1
Internal wiring termination 'z Vibrating cells
it connections
IvvvIIIII l."~~~~su~s~ distance element
Isolating bellow assembly
a. 4
*e- Weldneck process flange
Figure 8.1 5 Solartron 7835 liquid density transducer. Courtesy SolartronTransducers.
the constants in the associated readout or signal between the mass of a gas rn, its molecular weight
conditioning circuits. The density-frequency rela- Mw, and number of moles is given by
tion is given by
n = rnlMw
p=Ko($- 1) When the compressibility factor Z is 1.0 the gas is
called ideal or perfect. When the specific heat is
where p is the density of the measured fluid; KO is assumed to be only temperature dependent the
constant for the transducer. TO is the time period gas is referred to as ideal. If the ideal relative
of oscillation under vacuum conditions, and Tis density RD of a gas is defined as the ratio of
the time period of oscillation under operating molecular weight of the gas to that of air, then
conditions.
It is noteworthy that, although the relation
between density and the period of the oscillation
strictly obeys a square law, it is linear within whereas the real relative density is defined as the
2 percent for a change in density of 20 percent. ratio of the density of the gas to that of air, which
For narrower spans the error is proportionally is
smaller.
RD= !%?
Pair
8.6.2 Gas density measurements for a particular temperature and pressure.
The relationship between temperature, pressure, The above equation can be rearranged as a
and volume of a gas is given by density equation, thus
rn
PV = nZRo T p=-= SGMwairP
where P is the absolute pressure, Vis the volume, V ZRoT
and n is the number of moles. Z is the compres- Most relative density measuring instruments
sibility factor, Ro is the Universal gas constant, operate at pressures and temperatures close to
and Tis the absolute temperature. Use of the mole ambient conditions and hence measure real rela-
in this equation eliminates the need for determin- tive density rather than the ideal relative density
ing individual gas constants, and the relationship which is based on molecular weights and does not