Page 141 - Instrumentation Reference Book 3E
P. 141
Pressure measurement 125
I Applied I pressure
Atmospheric
Atmospheric
pressure
-
Unknown I ‘I
pressure I =A
cc
L level
‘Liquid density=p
Figure 9.2 Simple U-tube manometer. ‘Density p,
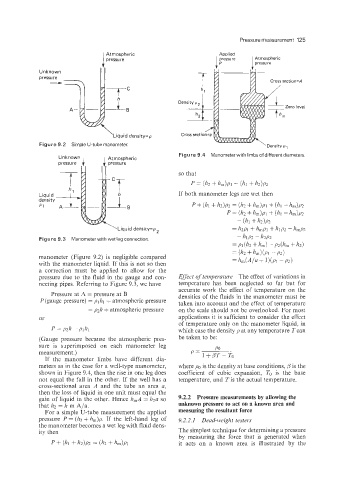
Unknown , I Atmospheric Figure 9.4 Manometer with limbs of different diameters.
so that
P = (122 + h,)m - (hl + h2)P2
Liquid If both manometer legs are wet then
density
P1 P + (hl + h2)PZ = (122 + h,)Pl + (121 - h,)p2
p = (h2 + h,)Pl + (121 - IZ,)PZ
- (!I1 + h2)P2
density=p2 = h2P1 + hmPl + hlP2 - I2,PZ
Figure 9.3 Manometer with wet leg connection. - hlP2 - h2PZ
= Pl(122 + hm) - P2(h, + 122)
= (h2 + h,)(Pl - P2)
manometer (Figure 9.2) is negligible compared
with the manometer liquid. If this is not so then = hm(A/a + l)(Pl - P2)
a correction must be applied to allow for the
pressure due to the fluid in the gauge and con- Effect of temperature The effect of variations in
necting pipes. Referring to Figure 9.3, we have temperature has been neglected so far but for
accurate work the effect of temperature on the
Pressure at A = pressure at B densities of the fluids in the manometer must be
P(gauge pressure) = plhl + atmospheric pressure taken into account and the effect of temperature
+
= p~h atmospheric pressure on the scale should not be overlooked. For most
or applications it is sufficient to consider the effect
of temperature only on the manometer liquid, in
P = p2h ~ plh, which case the density p at any temperature T can
(Gauge pressure because the atmospheric pres- be taken to be:
sure is saperimposed on each manometer leg PO
measurement.) P=l+pT-TO
If the manometer limbs have different dia-
meters as in the case for a well-type manometer, where is the density at base conditions, p is the
shown in Figure 9.4, then the rise in one leg does coefficient of cubic expansion, TO is the base
not equal the fall in the other. If the well has a temperature, and Tis the actual temperature.
cross-sectional area A and the tube an area a,
then the loss of liquid in one unit must equal the
gain of liquid in the other. Hence h,A = h2a so 9.2.2 Pressure measurements by allowing the
that h2 = lz m Ala. unknown pressure to act on a known area and
For a simple U-tube measurement the applied measuring the resultant force
pressure P = (h2 + h,)p. If the left-hand leg of 9.2.2.1 Dead-weight testers
the manometer becomes a wet leg with fluid dens-
ity then The simplest technique for determining a pressure
by measuring the force that is generated when
p + (hl + h2)P2 = (h2 + hnl)Pl it acts on a known area is illustrated by the