Page 347 - Instrumentation Reference Book 3E
P. 347
330 Chemical analysis: spectroscopy
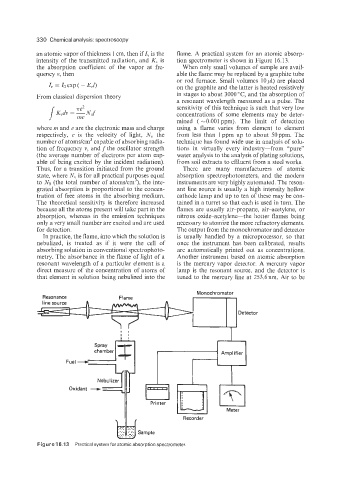
an atomic vapor of thickness 1 cm, then if Z, is the flame. A practical system for an atomic absorp-
intensity of the transmitted radiation, and K, is tion spectrometer is shown in Figure 16.13.
the absorption coefficient of the vapor at fre- When only small volumes of sample are avail-
quency v, then able the flame may be replaced by a graphite tube
or rod furnace. Small volumes lo$) are placed
Z, = IO exp ( - &I) on the graphite and the latter is heated resistively
From classical dispersion theory in stages to about 3000 “C, and the absorption of
a resonant wavelength measured as a pulse. The
sensitivity of this technique is such that very low
concentrations of some elements may be deter-
mined ( -0.001 ppm). The limit of detection
where m and e are the electronic mass and charge using a flame varies from element to element
respectively, c is the velocity of light, N, the from less than 1 ppm up to about 50ppm. The
number of atoms/cm3 capable of absorbing radia- technique has found wide use in analysis of solu-
tion of frequency v, and f the oscillator strength tions in virtually every industry-from “pure”
(the average number of electrons per atom cap- water analysis to the analysis of plating solutions,
able of being excited by the incident radiation). from soil extracts to effluent from a steel works.
Thus, for a transition initiated from the ground There are many manufacturers of atomic
state, where N, is for all practical pur oses equal absorption spectrophotometers, and the modern
P
to No (the total number of atomskm ), the inte- instruments are very highly automated. The reson-
grated absorption is proportional to the concen- ant line source is usually a high intensity hollow
tration of free atoms in the absorbing medium. cathode lamp and up to ten of these may be con-
The theoretical sensitivity is therefore increased tained in a turret so that each is used in turn. The
because all the atoms present will take part in the flames are usually air-propane, air-acetylene, or
absorption, whereas in the emission techniques nitrous oxide-acetylene-the hotter flames being
only a very small number are excited and are used necessary to atomize the more refractory elements.
for detection. The output from the monochromator and detector
In practice, the flame, into which the solution is is usually handled by a microprocessor, so that
nebulized, is treated as if it were the cell of once the instrument has been calibrated, results
absorbing solution in conventional spectrophoto- are automatically printed out as concentrations.
metry. The absorbance in the flame of light of a Another instrument based on atomic absorption
resonant wavelength of a particular element is a is the mercury vapor detector. A mercury vapor
direct measure of the concentration of atoms of lamp is the resonant source, and the detector is
that element in solution being nebulized into the tuned to the mercury line at 253.6nm. Air to be
Monochromator
Resonance Flame
Detector
I I I 1 I 8
Fuel - ~ Amplifier
chamber
Oxidant --+
Printer i I I
Meter
Recorder
Figure 1 6 .I 3 Practical system for atomic absorption spectrometer.