Page 352 - Instrumentation Reference Book 3E
P. 352
Microwave spectroscopy 335
4. Resonant cavity
with hole for
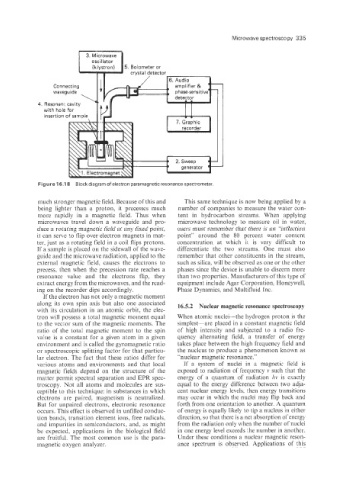
Figure 16.1 8 Block diagram of electron paramagnetic resonance spectrometer.
much stronger magnetic field. Because of this and This same technique is now being applied by a
being lighter than a proton, it precesses much number of companies to measure the water con-
more rapidly in a magnetic field. Thus when tent in hydrocarbon streams. When applying
microwaves travel down a waveguide and pro- microwave technology to measure oil in water,
duce a rotating magnetic field at any fixed point, users must remember that there is an “inflection
it can serve to flip over electron magnets in mat- point” around the 80 percent water content
ter, just as a rotating field in a coil flips protons. concentration at which it is very difficult to
If a sample is placed on the sidewall of the wave- differentiate the two streams. One must also
guide and the microwave radiation, applied to the remember that other constituents in the stream,
external magnetic field, causes the electrons to such as silica, will be observed as one or the other
precess, then when the precession rate reaches a phases since the device is unable to discern more
resonance value and the electrons flip, they than two properties. Manufacturers of this type of
extract energy from the microwaves, and the read- equipment include Agar Corporation, Honeywell,
ing on the recorder dips accordingly. Phase Dynamics. and Multifluid Inc.
If the electron has not only a magnetic moment
along its own spin axis but also one associated 16.5.2 Nuclear magnetic resonance spectroscopy
with its circulation in an atomic orbit, the elec-
tron will possess a total magnetic moment equal When atomic nuclei-the hydrogen proton is the
to the vec:or sum of the magnetic moments. The simplest-are placed in a constant magnetic field
ratio of the total magnetic moment to the spin of high intensity and subjected to a radio fre-
value is a constant for a given atom in a given quency alternating field, a transfer of energy
environment and is called the gyromagnetic ratio takes place between the high frequency field and
or spectroscopic splitting factor for that particu- the nucleus to produce a phenomenon known as
lar electron. The fact that these ratios differ for “nuclear magnetic resonance.”
various atoms and environments and that local If a system of nuclei in a magnetic field is
magnetic fields depend on the structure of the exposed to radiation of frequency v such that the
matter permit spectral separation and EPR spec- energy of a quantum of radiation IIV is exactly
troscopy. Not all atoms and molecules are sus- equal to the energy difference between two adja-
ceptible to this technique: in substances in which cent nuclear energy levels, then energy transitions
electrons are paired. magnetism is neutralized. may occur in which the nuclei may flip back and
But for unpaired electrons, electronic resonance forth from one orientation to another. A quantum
occurs. This effect is observed in unfilled conduc- of energy is equally likely to tip a nucleus in either
tion bands, transition element ions, free radicals, direction, so that there is a net absorption of energy
and impurities in semiconductors, and, as might from the radiation only when the number of nuclei
be expected, applications in the biological field in one energy level exceeds the number in another.
are fruitful. The most common use is the para- Under these conditions a nuclear magnetic reson-
magnetic oxygen analyzer. ance spectrum is observed. Applications of this