Page 116 - Introduction to Computational Fluid Dynamics
P. 116
P1: IWV
0 521 85326 5
CB908/Date
0521853265c04
4.10 APPLICATIONS
(b)
(a) May 25, 2005 11:7 95
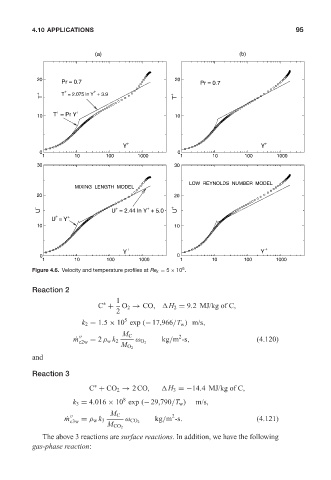
20 Pr = 0.7 20 Pr = 0.7
+ +
T + T = 2.075 ln Y + 3.9 T +
+ +
10 T = Pr Y 10
+ +
Y Y
0 0
1 10 100 1000 1 10 100 1000
30 30
LOW REYNOLDS NUMBER MODEL
MIXING LENGTH MODEL
20 20
+
+
U + U = 2.44 ln Y + 5.0 U +
+ +
U = Y
10 10
+ +
Y Y
0 0
1 10 100 1000 1 10 100 1000
6
Figure 4.6. Velocity and temperature profiles at Re x = 5 × 10 .
Reaction 2
1
∗
C + O 2 → CO, H 2 = 9.2 MJ/kg of C,
2
5
k 2 = 1.5 × 10 exp (−17,966/T w ) m/s,
M C 2
˙ m kg/m -s, (4.120)
c2w = 2ρ w k 2 ω O 2
M O 2
and
Reaction 3
∗
C + CO 2 → 2CO, H 3 =−14.4 MJ/kg of C,
8
k 3 = 4.016 × 10 exp (−29,790/T w ) m/s,
M C 2
˙ m c3w = ρ w k 3 ω CO 2 kg/m -s. (4.121)
M CO 2
The above 3 reactions are surface reactions. In addition, we have the following
gas-phase reaction: