Page 168 - Sami Franssila Introduction to Microfabrication
P. 168
Thermal Oxidation 147
15:29:15 13-FEB-3 15:25:26 13-FEB-3
10 16 SiO 2 Oxthi = 0.4097 Boron 10 16 SiO 2 Oxthi = 0.4097 Boron
10 15 10 15
Concentration (cm −3 ) 10 13 Concentration (cm −3 ) 10 13
14
14
10
10
12
12
10
10 11 10 11
10
10 10 10 10
0.00 0.20 0.40 0.60 0.80 1.00 1.20 0.00 0.20 0.40 0.60 0.80 1.00 1.20
Depth (µm) Depth (µm)
(a) (b)
◦
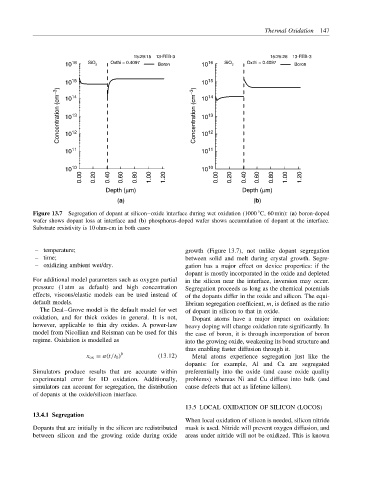
Figure 13.7 Segregation of dopant at silicon–oxide interface during wet oxidation (1000 C, 60 min): (a) boron-doped
wafer shows dopant loss at interface and (b) phosphorus-doped wafer shows accumulation of dopant at the interface.
Substrate resistivity is 10 ohm-cm in both cases
– temperature; growth (Figure 13.7), not unlike dopant segregation
– time; between solid and melt during crystal growth. Segre-
– oxidizing ambient wet/dry. gation has a major effect on device properties: if the
dopant is mostly incorporated in the oxide and depleted
For additional model parameters such as oxygen partial in the silicon near the interface, inversion may occur.
pressure (1 atm as default) and high concentration Segregation proceeds as long as the chemical potentials
effects, viscous/elastic models can be used instead of of the dopants differ in the oxide and silicon. The equi-
default models. librium segregation coefficient, m, is defined as the ratio
The Deal–Grove model is the default model for wet of dopant in silicon to that in oxide.
oxidation, and for thick oxides in general. It is not, Dopant atoms have a major impact on oxidation:
however, applicable to thin dry oxides. A power-law heavy doping will change oxidation rate significantly. In
model from Nicollian and Reisman can be used for this the case of boron, it is through incorporation of boron
regime. Oxidation is modelled as into the growing oxide, weakening its bond structure and
thus enabling faster diffusion through it.
x ox = a(t/t 0 ) b (13.12) Metal atoms experience segregation just like the
dopants: for example, Al and Ca are segregated
Simulators produce results that are accurate within preferentially into the oxide (and cause oxide quality
experimental error for 1D oxidation. Additionally, problems) whereas Ni and Cu diffuse into bulk (and
simulators can account for segregation, the distribution cause defects that act as lifetime killers).
of dopants at the oxide/silicon interface.
13.5 LOCAL OXIDATION OF SILICON (LOCOS)
13.4.1 Segregation
When local oxidation of silicon is needed, silicon nitride
Dopants that are initially in the silicon are redistributed mask is used. Nitride will prevent oxygen diffusion, and
between silicon and the growing oxide during oxide areas under nitride will not be oxidized. This is known