Page 61 - Lindens Handbook of Batteries
P. 61
2.18 PRINCIPLES OF OPERATION
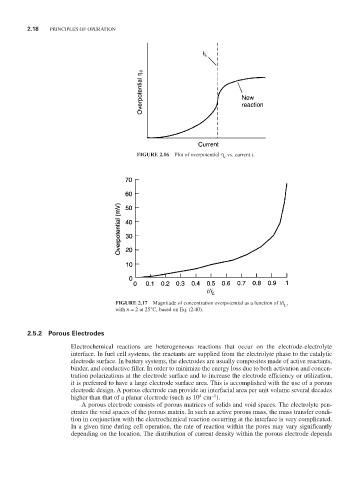
FIGURE 2.16 Plot of overpotential η vs. current i.
c
FIGURE 2.17 Magnitude of concentration overpotential as a function of i/i ,
L
with n = 2 at 25°C, based on Eq. (2.40).
2.5.2 Porous Electrodes
Electrochemical reactions are heterogeneous reactions that occur on the electrode-electrolyte
interface. In fuel cell systems, the reactants are supplied from the electrolyte phase to the catalytic
electrode surface. In battery systems, the electrodes are usually composites made of active reactants,
binder, and conductive filler. In order to minimize the energy loss due to both activation and concen-
tration polarizations at the electrode surface and to increase the electrode efficiency or utilization,
it is preferred to have a large electrode surface area. This is accomplished with the use of a porous
electrode design. A porous electrode can provide an interfacial area per unit volume several decades
-1
4
higher than that of a planar electrode (such as 10 cm ).
A porous electrode consists of porous matrices of solids and void spaces. The electrolyte pen-
etrates the void spaces of the porous matrix. In such an active porous mass, the mass transfer condi-
tion in conjunction with the electrochemical reaction occurring at the interface is very complicated.
In a given time during cell operation, the rate of reaction within the pores may vary significantly
depending on the location. The distribution of current density within the porous electrode depends