Page 64 - Lindens Handbook of Batteries
P. 64
ELECTROCHEMICAL PRINCIPLES AND REACTIONS 2.21
Equation (2.41) should really be written in a form that describes these two components. Equation (2.44)
shows such a modification,
+ ν
EE - i tr i + ( f i ) (2.44)
=
c
where r = cell resistance, i = faradaic current, and i = capacity current.
f
c
At small values of voltage sweep rate, typically below 1 mV/s, the capacity effects are small and
in most cases can be ignored. At greater values of sweep rate, a connection needs to be applied to
17
interpretations of i as described by Nicholson and Shain. With regard to the correction for ohmic
p
drop in solution, typically this can be handled adequately by careful cell design and positive feed-
back compensation circuitry in the electronic instrumentation.
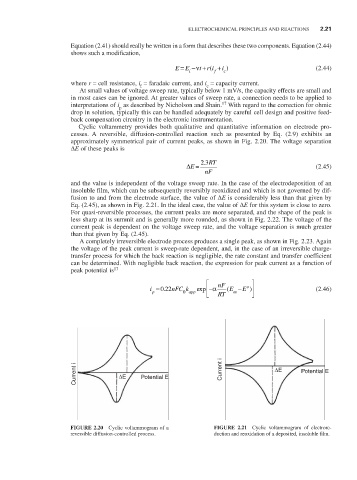
Cyclic voltammetry provides both qualitative and quantitative information on electrode pro-
cesses. A reversible, diffusion-controlled reaction such as presented by Eq. (2.9) exhibits an
approximately symmetrical pair of current peaks, as shown in Fig. 2.20. The voltage separation
∆E of these peaks is
.
∆E = 23 RT (2.45)
nF
and the value is independent of the voltage sweep rate. In the case of the electrodeposition of an
insoluble film, which can be subsequently reversibly reoxidized and which is not governed by dif-
fusion to and from the electrode surface, the value of ∆E is considerably less than that given by
Eq. (2.45), as shown in Fig. 2.21. In the ideal case, the value of ∆E for this system is close to zero.
For quasi-reversible processes, the current peaks are more separated, and the shape of the peak is
less sharp at its summit and is generally more rounded, as shown in Fig. 2.22. The voltage of the
current peak is dependent on the voltage sweep rate, and the voltage separation is much greater
than that given by Eq. (2.45).
A completely irreversible electrode process produces a single peak, as shown in Fig. 2.23. Again
the voltage of the peak current is sweep-rate dependent, and, in the case of an irreversible charge-
transfer process for which the back reaction is negligible, the rate constant and transfer coefficient
can be determined. With negligible back reaction, the expression for peak current as a function of
peak potential is 17
nF
o
i = . nFC k exp α ( E - E ) (2.46)
- 022
p 0 app m
RT
Current i ∆E Potential E Current i ∆E Potential E
FIGURE 2.20 Cyclic voltammogram of a FIGURE 2.21 Cyclic voltammogram of electrore-
reversible diffusion-controlled process. duction and reoxidation of a deposited, insoluble film.