Page 65 - Lindens Handbook of Batteries
P. 65
2.22 PRINCIPLES OF OPERATION
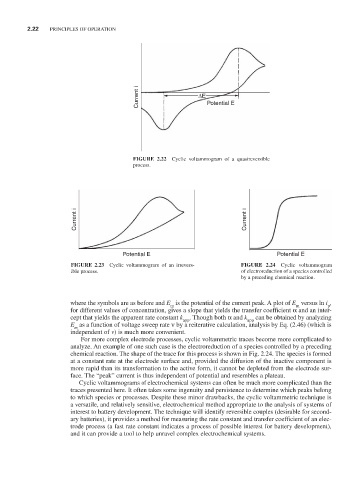
Current i ∆E Potential E
FIGURE 2.22 Cyclic voltammogram of a quasireversible
process.
Current i Current i
Potential E Potential E
FIGURE 2.23 Cyclic voltammogram of an irrevers- FIGURE 2.24 Cyclic voltammogram
ible process. of electroreduction of a species controlled
by a preceding chemical reaction.
where the symbols are as before and E is the potential of the current peak. A plot of E versus ln i ,
m
m
p
for different values of concentration, gives a slope that yields the transfer coefficient α and an inter-
cept that yields the apparent rate constant k app . Though both α and k app can be obtained by analyzing
E as a function of voltage sweep rate ν by a reiterative calculation, analysis by Eq. (2.46) (which is
m
independent of v) is much more convenient.
For more complex electrode processes, cyclic voltammetric traces become more complicated to
analyze. An example of one such case is the electroreduction of a species controlled by a preceding
chemical reaction. The shape of the trace for this process is shown in Fig. 2.24. The species is formed
at a constant rate at the electrode surface and, provided the diffusion of the inactive component is
more rapid than its transformation to the active form, it cannot be depleted from the electrode sur-
face. The “peak” current is thus independent of potential and resembles a plateau.
Cyclic voltammograms of electrochemical systems can often be much more complicated than the
traces presented here. It often takes some ingenuity and persistence to determine which peaks belong
to which species or processes. Despite these minor drawbacks, the cyclic voltammetric technique is
a versatile, and relatively sensitive, electrochemical method appropriate to the analysis of systems of
interest to battery development. The technique will identify reversible couples (desirable for second-
ary batteries), it provides a method for measuring the rate constant and transfer coefficient of an elec-
trode process (a fast rate constant indicates a process of possible interest for battery development),
and it can provide a tool to help unravel complex electrochemical systems.