Page 67 - Lindens Handbook of Batteries
P. 67
2.24 PRINCIPLES OF OPERATION
Potential Potential E τ/4
Transition
time τ
τ/4 Time τ Time
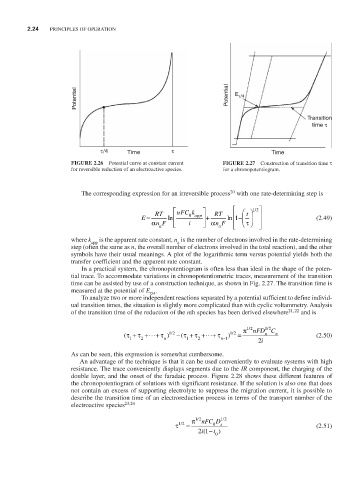
FIGURE 2.26 Potential curve at constant current FIGURE 2.27 Construction of transition time τ
for reversible reduction of an electroactive species. for a chronopotentiogram.
20
The corresponding expression for an irreversible process with one rate-determining step is
nFCk 1//2
t
E = RT ln 0 app + RT ln 1 - (2.49)
α nF i α nF τ
a
a
where k app is the apparent rate constant, n is the number of electrons involved in the rate-determining
a
step (often the same as n, the overall number of electrons involved in the total reaction), and the other
symbols have their usual meanings. A plot of the logarithmic term versus potential yields both the
transfer coefficient and the apparent rate constant.
In a practical system, the chronopotentiogram is often less than ideal in the shape of the poten-
tial trace. To accommodate variations in chronopotentiometric traces, measurement of the transition
time can be assisted by use of a construction technique, as shown in Fig. 2.27. The transition time is
measured at the potential of E .
τ/4
To analyze two or more independent reactions separated by a potential sufficient to define individ-
ual transition times, the situation is slightly more complicated than with cyclic voltammetry. Analysis
of the transition time of the reduction of the nth species has been derived elsewhere 21, 22 and is
1//2
/ 12
n
( τ τ + ⋅⋅⋅+ τ + ) / 12 ( τ + τ - τ +⋅⋅⋅+ ) / 12 = π nFD C n (2.50)
1 2 n 1 2 n 1 - 2i
As can be seen, this expression is somewhat cumbersome.
An advantage of the technique is that it can be used conveniently to evaluate systems with high
resistance. The trace conveniently displays segments due to the IR component, the charging of the
double layer, and the onset of the faradaic process. Figure 2.28 shows these different features of
the chronopotentiogram of solutions with significant resistance. If the solution is also one that does
not contain an excess of supporting electrolyte to suppress the migration current, it is possible to
describe the transition time of an electroreduction process in terms of the transport number of the
electroactive species 23,24
/
12
/
/
0
s
τ 12 = π nFCD 12 (2.51)
i
21- t )
(
0