Page 72 - Lindens Handbook of Batteries
P. 72
ELECTROCHEMICAL PRINCIPLES AND REACTIONS 2.29
34
such as the Galvanostatic Intermittent Titration Technique (GITT) and Potentiostatic Intermittent
35
Titration Technique (PITT) offer more direct determination of kinetic and thermodynamic prop-
erties of single and multiphase electrodes (e.g., alloys and Li-insertion materials). Both methods
enable one to determine the phase diagram of an electrode material as a function of capacity and
voltage. For example, in a two-phase material at equilibrium at constant temperature and pressure,
the potential of the electrode material will be independent of composition. However, when a phase
transition occurs such as from a two-phase to single phase domain, the electrode potential will now
vary as a function of composition. In addition, both GITT and PITT methods are convenient for
determining the diffusion coefficient of the migrating ion in the various phases of the solid-state
material. Solid-state diffusion coefficients can be determined from the Warburg impedance in EIS
measurements as described above, and agreement is dependent upon the equivalent circuit model
used in the deconvolution of the EIS data. Thus determination of the phases and diffusion coef-
ficients by GITT and PITT are helpful in modeling equivalent circuits. Essentials of the GITT and
PITT techniques are given below.
Galvanostatic Intermittent Titration Technique (GITT). The GITT method is a form of chro-
nopotentiometry (discussed above), but it is simpler in determining diffusion coefficient for ions
intercalating and deintercalating into and out of composite electrode material, i.e., materials based
on at least two components. In this transient method, a constant current pulse is sequentially applied
to the electrode for a given time t to remove or insert about 2 to 5% of the electrode’s total capacity,
e.g., x in Li CoO . The capacity inserted or deinserted is simply it (current vs. time), and the total
2
x
number of constant current steps required to fully cover the stable range of x will depend upon the
amount of capacity added to or removed from the electrode material from each constant current step.
After each defined constant current pulse, the electrode potential is allowed to rest until it reaches a
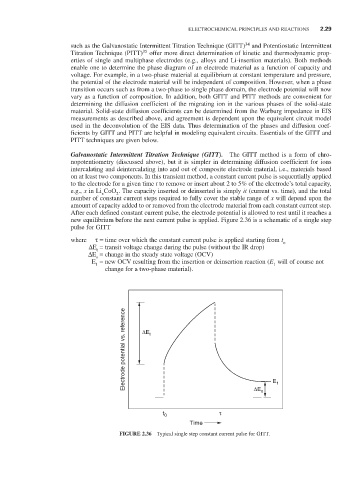
new equilibrium before the next current pulse is applied. Figure 2.36 is a schematic of a single step
pulse for GITT
where τ = time over which the constant current pulse is applied starting from t o
∆ E = transit voltage change during the pulse (without the IR drop)
t
∆ E = change in the steady state voltage (OCV)
s
E = new OCV resulting from the insertion or deinsertion reaction (E will of course not
1
1
change for a two-phase material).
Electrode potential vs. reference ∆E t E
t 0 τ ∆E s 1
Time
FIGURE 2.36 Typical single step constant current pulse for GITT.