Page 73 - Lindens Handbook of Batteries
P. 73
2.30 PRINCIPLES OF OPERATION
4.4
4.2
4.0
Potential vs. Li/Li + /V 3.6
3.8
3.4
3.2
3.0
2.8
0.2 0.4 0.6 0.8 1.0
x in Li CoO 2
X
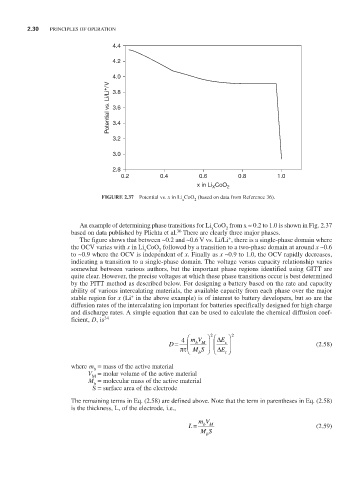
FIGURE 2.37 Potential vs. x in Li CoO (based on data from Reference 36).
x 2
An example of determining phase transitions for Li CoO from x ≈ 0.2 to 1.0 is shown in Fig. 2.37
2
x
based on data published by Plichta et al. There are clearly three major phases.
36
+
The figure shows that between ~0.2 and ~0.6 V vs. Li/Li , there is a single-phase domain where
the OCV varies with x in Li CoO followed by a transition to a two-phase domain at around x ~0.6
2
x
to ~0.9 where the OCV is independent of x. Finally as x ~0.9 to 1.0, the OCV rapidly decreases,
indicating a transition to a single-phase domain. The voltage versus capacity relationship varies
somewhat between various authors, but the important phase regions identified using GITT are
quite clear. However, the precise voltages at which these phase transitions occur is best determined
by the PITT method as described below. For designing a battery based on the rate and capacity
ability of various intercalating materials, the available capacity from each phase over the major
+
stable region for x (Li in the above example) is of interest to battery developers, but so are the
diffusion rates of the intercalating ion important for batteries specifically designed for high charge
and discharge rates. A simple equation that can be used to calculate the chemical diffusion coef-
ficient, D, is 34
2
2
bM
D = 4 mV ∆ E s (2.58)
πτ MS ∆ E t
b
where m = mass of the active material
b
V = molar volume of the active material
M
M = molecular mass of the active material
b
S = surface area of the electrode
The remaining terms in Eq. (2.58) are defined above. Note that the term in parentheses in Eq. (2.58)
is the thickness, L, of the electrode, i.e.,
mV
L = bM (2.59)
MS
b