Page 76 - Lindens Handbook of Batteries
P. 76
ELECTROCHEMICAL PRINCIPLES AND REACTIONS 2.33
where M represents carbon or graphite (LiC ), or a metal oxide, such as Li CoO . In general, the
2
x
6
electrode reaction can be written as
M + Li + x + e x - Li M (2.63)
x
The Gibbs energies as a function of lithium content (x) is related to the OCV and state functions by
∆ FE x()= ∆Gx ()=- H x() - T ∆Sx() (2.64)
where
δ E
F
∆Sx ()= (2.65)
δ T x
and
δ E
∆Hx ()=- FE x ()+ TF (2.66)
δ T x
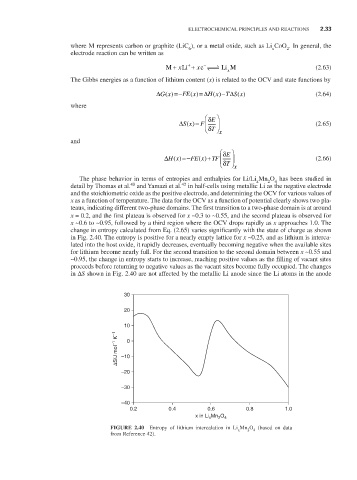
The phase behavior in terms of entropies and enthalpies for Li/Li Mn O has been studied in
x
4
2
40
detail by Thomas et al. and Yamazi et al. in half-cells using metallic Li as the negative electrode
42
and the stoichiometric oxide as the positive electrode, and determining the OCV for various values of
x as a function of temperature. The data for the OCV as a function of potential clearly shows two pla-
teaus, indicating different two-phase domains. The first transition to a two-phase domain is at around
x = 0.2, and the first plateau is observed for x ~0.3 to ~0.55, and the second plateau is observed for
x ~0.6 to ~0.95, followed by a third region where the OCV drops rapidly as x approaches 1.0. The
change in entropy calculated from Eq. (2.65) varies significantly with the state of charge as shown
in Fig. 2.40. The entropy is positive for a nearly empty lattice for x ~0.25, and as lithium is interca-
lated into the host oxide, it rapidly decreases, eventually becoming negative when the available sites
for lithium become nearly full. For the second transition to the second domain between x ~0.55 and
~0.95, the change in entropy starts to increase, reaching positive values as the filling of vacant sites
proceeds before returning to negative values as the vacant sites become fully occupied. The changes
in ∆S shown in Fig. 2.40 are not affected by the metallic Li anode since the Li atoms in the anode
30
20
10
∆S/J mol –1 K –1 –10 0
–20
–30
–40
0.2 0.4 0.6 0.8 1.0
x in Li x Mn 2 O 4
FIGURE 2.40 Entropy of lithium intercalation in Li Mn O (based on data
x
4
2
from Reference 42).