Page 77 - Lindens Handbook of Batteries
P. 77
2.34 PRINCIPLES OF OPERATION
41
are in the same environment at any state of charge. The maximum for positive values of ∆S in
phase diagrams as a function of x in Li M can also be correlated with higher diffusion coefficients
x
and interstitial distances in the different phases as determined from crystal structures derived from
39
X-ray diffraction data.
2.6.6 Electrodes
Several electrochemical techniques have been discussed in the preceding sections, but little was
mentioned about electrodes or electrode geometries used in the various measurements. This section
deals with electrodes and electrode systems. A basic two-electrode cell contains an anode (negative
electrode) and a cathode (positive electrode) and is typically used to study individual battery cell
properties to determine Gibbs energies, energy densities, and specific energies. For studying the
properties of a given electrode material by cyclic voltammetry and transient techniques, a three-
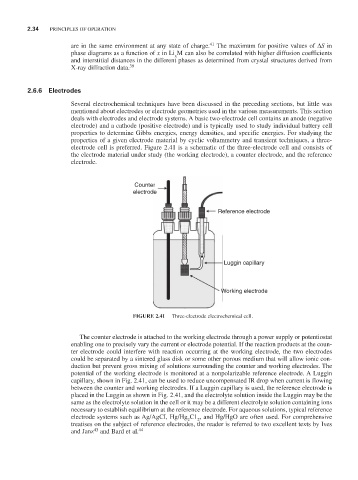
electrode cell is preferred. Figure 2.41 is a schematic of the three-electrode cell and consists of
the electrode material under study (the working electrode), a counter electrode, and the reference
electrode.
Counter
electrode
Reference electrode
Luggin capillary
Working electrode
FIGURE 2.41 Three-electrode electrochemical cell.
The counter electrode is attached to the working electrode through a power supply or potentiostat
enabling one to precisely vary the current or electrode potential. If the reaction products at the coun-
ter electrode could interfere with reaction occurring at the working electrode, the two electrodes
could be separated by a sintered glass disk or some other porous medium that will allow ionic con-
duction but prevent gross mixing of solutions surrounding the counter and working electrodes. The
potential of the working electrode is monitored at a nonpolarizable reference electrode. A Luggin
capillary, shown in Fig. 2.41, can be used to reduce uncompensated IR drop when current is flowing
between the counter and working electrodes. If a Luggin capillary is used, the reference electrode is
placed in the Luggin as shown in Fig. 2.41, and the electrolyte solution inside the Luggin may be the
same as the electrolyte solution in the cell or it may be a different electrolyte solution containing ions
necessary to establish equilibrium at the reference electrode. For aqueous solutions, typical reference
electrode systems such as Ag/AgCI, Hg/Hg C1 , and Hg/HgO are often used. For comprehensive
2
2
treatises on the subject of reference electrodes, the reader is referred to two excellent texts by Ives
43
and Janz and Bard et al. 44