Page 100 - Macromolecular Crystallography
P. 100
ISOMORPHOUS REPLACEMENT 89
(a)
Imaginary F PH F H Imaginary F
F P PH F P
α Pa
α
P
F H α Pb Real
Real F P
F PH
(b)
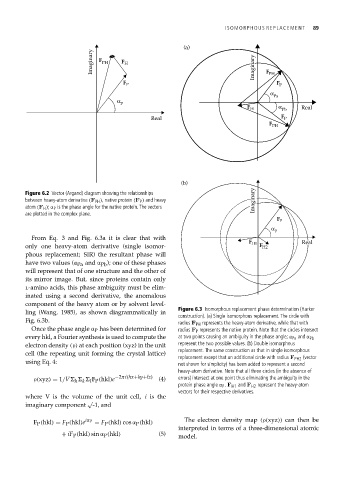
Figure 6.2 Vector (Argand) diagram showing the relationships
between heavy-atom derivative (F PH ), native protein (F P ) and heavy Imaginary
atom (F H ); α P is the phase angle for the native protein. The vectors
are plotted in the complex plane.
F P
α
P
From Eq. 3 and Fig. 6.3a it is clear that with
F H1 Real
only one heavy-atom derivative (single isomor- F H2
phous replacement; SIR) the resultant phase will
have two values (α Pa and α Pb ); one of these phases
will represent that of one structure and the other of
its mirror image. But, since proteins contain only
l-amino acids, this phase ambiguity must be elim-
inated using a second derivative, the anomalous
component of the heavy atom or by solvent level-
Figure 6.3 Isomorphous replacement phase determination (Harker
ling (Wang, 1985), as shown diagrammatically in
construction). (a) Single isomorphous replacement. The circle with
Fig. 6.3b. radius F PH represents the heavy-atom derivative, while that with
Once the phase angle α P has been determined for radius F P represents the native protein. Note that the circles intersect
every hkl, a Fourier synthesis is used to compute the at two points causing an ambiguity in the phase angle; α Pa and α Pb
electron density (ρ) at each position (xyz) in the unit represent the two possible values. (b) Double isomorphous
replacement. The same construction as that in single isomorphous
cell (the repeating unit forming the crystal lattice)
replacement except that an additional circle with radius F PH2 (vector
using Eq. 4: not shown for simplicity) has been added to represent a second
heavy-atom derivative. Note that all three circles (in the absence of
ρ(xyz) = 1/V F P (hkl)e −2πi(hx+ky+lz) (4) errors) intersect at one point thus eliminating the ambiguity in the
h k
l
protein phase angle α P . F H1 and F H2 represent the heavy-atom
vectors for their respective derivatives.
where V is the volume of the unit cell, i is the
√
imaginary component -1, and
The electron density map (ρ(xyz)) can then be
F P (hkl) = F P (hkl)e iα P = F P (hkl) cos α P (hkl)
interpreted in terms of a three-dimensional atomic
+ iF P (hkl) sin α P (hkl) (5)
model.