Page 303 - Managing Global Warming
P. 303
Methane hydrate as a “new energy” 263
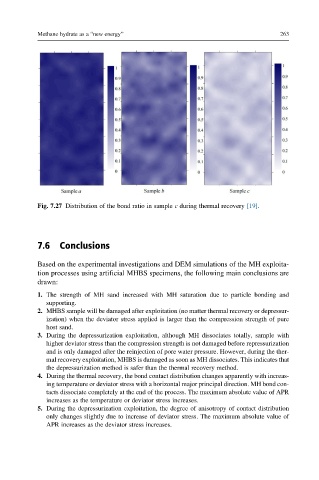
Fig. 7.27 Distribution of the bond ratio in sample c during thermal recovery [19].
7.6 Conclusions
Based on the experimental investigations and DEM simulations of the MH exploita-
tion processes using artificial MHBS specimens, the following main conclusions are
drawn:
1. The strength of MH sand increased with MH saturation due to particle bonding and
supporting.
2. MHBS sample will be damaged after exploitation (no matter thermal recovery or depressur-
ization) when the deviator stress applied is larger than the compression strength of pure
host sand.
3. During the depressurization exploitation, although MH dissociates totally, sample with
higher deviator stress than the compression strength is not damaged before repressurization
and is only damaged after the reinjection of pore water pressure. However, during the ther-
mal recovery exploitation, MHBS is damaged as soon as MH dissociates. This indicates that
the depressurization method is safer than the thermal recovery method.
4. During the thermal recovery, the bond contact distribution changes apparently with increas-
ing temperature or deviator stress with a horizontal major principal direction. MH bond con-
tacts dissociate completely at the end of the process. The maximum absolute value of APR
increases as the temperature or deviator stress increases.
5. During the depressurization exploitation, the degree of anisotropy of contact distribution
only changes slightly due to increase of deviator stress. The maximum absolute value of
APR increases as the deviator stress increases.