Page 473 - 04. Subyek Engineering Materials - Manufacturing, Engineering and Technology SI 6th Edition - Serope Kalpakjian, Stephen Schmid (2009)
P. 473
Section 17.4 Sintering 453
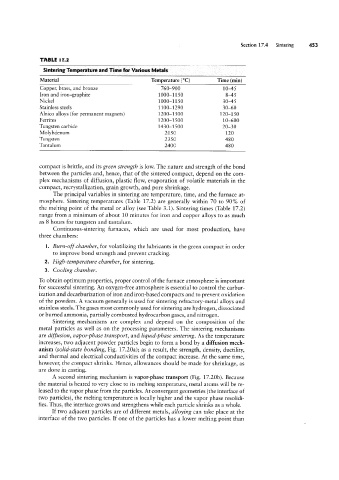
TABLE l7.2
Sintering Temperature and Time for Various Metals
Material Temperature (°C) Time (min)
Copper, brass, and bronze 760 900 10-45
Iron and iron-graphite 1000-1150 8-45
Nickel 1000-1 150 30-45
Stainless steels 1 100-1290 30-60
Alnico alloys (for permanent magnets) 1200-1300 120-150
Ferrites 1200 1500 10-600
Tungsten carbide 1430-1500 20-30
Molybdenum 2050 120
Tungsten 2350 480
Tantalum 2400 480
compact is brittle, and its green strength is low. The nature and strength of the bond
between the particles and, hence, that of the sintered compact, depend on the com-
plex mechanisms of diffusion, plastic flow, evaporation of volatile materials in the
compact, recrystallization, grain growth, and pore shrinkage.
The principal variables in sintering are temperature, time, and the furnace at-
mosphere. Sintering temperatures (Table 17.2) are generally within 70 to 90% of
the melting point of the metal or alloy (see Table 3.1). Sintering times (Table 17.2)
range from a minimum of about 10 minutes for iron and copper alloys to as much
as 8 hours for tungsten and tantalum.
Continuous-sintering furnaces, which are used for most production, have
three chambers:
l. Burn-off chaniher, for volatilizing the lubricants in the green compact in order
to improve bond strength and prevent cracking.
2. High-temperature chaniher, for sintering.
3. Cooling charnher.
To obtain optimum properties, proper control of the furnace atmosphere is important
for successful sintering. An oxygen-free atmosphere is essential to control the carbur-
ization and decarburization of iron and iron-based compacts and to prevent oxidation
of the powders. A vacuum generally is used for sintering refractory-metal alloys and
stainless steels. The gases most commonly used for sintering are hydrogen, dissociated
or burned ammonia, partially combusted hydrocarbon gases, and nitrogen.
Sintering mechanisms are complex and depend on the composition of the
metal particles as well as on the processing parameters. The sintering mechanisms
are diffusion, vapor-phase transport, and liquid-phase sintering. As the temperature
increases, two adjacent powder particles begin to form a bond by a diffusion mech-
anism (solid-state honding, Fig. 17.20a); as a result, the strength, density, ductility,
and thermal and electrical conductivities of the compact increase. At the same time,
however, the compact shrinks. Hence, allowances should be made for shrinkage, as
are done in casting.
A second sintering mechanism is vapor-phase transport (Fig. 17.20b). Because
the material is heated to very close to its melting temperature, metal atoms will be re-
leased to the vapor phase from the particles. At convergent geometries (the interface of
two particles), the melting temperature is locally higher and the vapor phase resolidi-
fies. Thus, the interface grows and strengthens while each particle shrinks as a whole.
If two adjacent particles are of different metals, alloying can take place at the
interface of the two particles. If one of the particles has a lower melting point than