Page 178 - Materials Science and Engineering An Introduction
P. 178
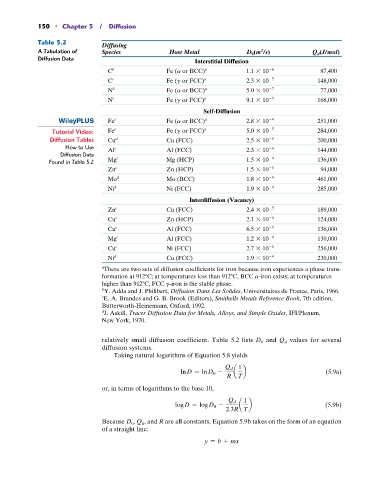
150 • Chapter 5 / Diffusion
Table 5.2
Diffusing
A Tabulation of Species Host Metal D 0 (m /s) Q d (J/mol)
2
Diffusion Data
Interstitial Diffusion
C b Fe (a or BCC) a 1.1 10 6 87,400
C c Fe (g or FCC) a 2.3 10 5 148,000
N b Fe (a or BCC) a 5.0 10 7 77,000
N c Fe (g or FCC) a 9.1 10 5 168,000
Self-Diffusion
Fe c Fe (a or BCC) a 2.8 10 4 251,000
Tutorial Video: Fe c Fe (g or FCC) a 5.0 10 5 284,000
Diffusion Tables Cu d Cu (FCC) 2.5 10 5 200,000
How to Use Al c Al (FCC) 2.3 10 4 144,000
Diffusion Data 4
Found in Table 5.2 Mg c Mg (HCP) 1.5 10 136,000
Zn c Zn (HCP) 1.5 10 5 94,000
Mo d Mo (BCC) 1.8 10 4 461,000
Ni d Ni (FCC) 1.9 10 4 285,000
Interdiffusion (Vacancy)
Zn c Cu (FCC) 2.4 10 5 189,000
Cu c Zn (HCP) 2.1 10 4 124,000
Cu c Al (FCC) 6.5 10 5 136,000
Mg c Al (FCC) 1.2 10 4 130,000
Cu c Ni (FCC) 2.7 10 5 256,000
Ni d Cu (FCC) 1.9 10 4 230,000
There are two sets of diffusion coefficients for iron because iron experiences a phase trans-
a
formation at 912 C; at temperatures less than 912 C, BCC a-iron exists; at temperatures
higher than 912 C, FCC g-iron is the stable phase.
Y. Adda and J. Philibert, Diffusion Dans Les Solides, Universitaires de France, Paris, 1966.
b
E. A. Brandes and G. B. Brook (Editors), Smithells Metals Reference Book, 7th edition,
c
Butterworth-Heinemann, Oxford, 1992.
J. Askill, Tracer Diffusion Data for Metals, Alloys, and Simple Oxides, IFI/Plenum,
d
New York, 1970.
values for several
relatively small diffusion coefficient. Table 5.2 lists D 0 and Q d
diffusion systems.
Taking natural logarithms of Equation 5.8 yields
Q d 1
ln D = ln D 0 - a b (5.9a)
R T
or, in terms of logarithms to the base 10,
1
log D = log D 0 - Q d a b (5.9b)
2.3R T
Because D 0 , Q d , and R are all constants, Equation 5.9b takes on the form of an equation
of a straight line:
y = b + mx