Page 358 - Materials Science and Engineering An Introduction
P. 358
330 • Chapter 9 / Phase Diagrams
2200
Hf
2000 L
Temperature (°C) 1800
1600
1400 V
HfV
1200 2
Hf
1000
0 20 40 60 80 100
(Hf) Composition (wt% V) (V)
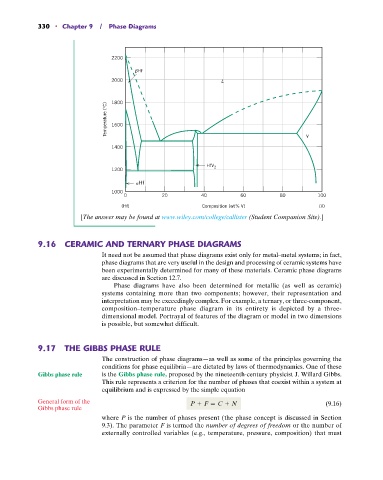
[The answer may be found at www.wiley.com/college/callister (Student Companion Site).]
9.16 CERAMIC AND TERNARY PHASE DIAGRAMS
It need not be assumed that phase diagrams exist only for metal–metal systems; in fact,
phase diagrams that are very useful in the design and processing of ceramic systems have
been experimentally determined for many of these materials. Ceramic phase diagrams
are discussed in Section 12.7.
Phase diagrams have also been determined for metallic (as well as ceramic)
systems containing more than two components; however, their representation and
interpretation may be exceedingly complex. For example, a ternary, or three-component,
composition–temperature phase diagram in its entirety is depicted by a three-
dimensional model. Portrayal of features of the diagram or model in two dimensions
is possible, but somewhat difficult.
9.17 THE GIBBS PHASE RULE
The construction of phase diagrams—as well as some of the principles governing the
conditions for phase equilibria—are dictated by laws of thermodynamics. One of these
Gibbs phase rule is the Gibbs phase rule, proposed by the nineteenth-century physicist J. Willard Gibbs.
This rule represents a criterion for the number of phases that coexist within a system at
equilibrium and is expressed by the simple equation
General form of the P + F = C + N (9.16)
Gibbs phase rule
where P is the number of phases present (the phase concept is discussed in Section
9.3). The parameter F is termed the number of degrees of freedom or the number of
externally controlled variables (e.g., temperature, pressure, composition) that must