Page 355 - Materials Science and Engineering An Introduction
P. 355
9.13 Equilibrium Diagrams Having Intermediate Phases or Compounds • 327
Composition (at% Pb)
0 5 10 20 30 40 70 100
700
L
1200
L
600
+
Mg Pb M
+ L 2
1000
500
Temperature (°C) 400 Mg Pb 800 Temperature (°F)
L
+
+
L
2
600
300
200 400
+ Mg Pb
2
+
100 Mg Pb 200
2
Pb
Mg 2
0
0 20 40 60 80 100
(Mg) Composition (wt% Pb) (Pb)
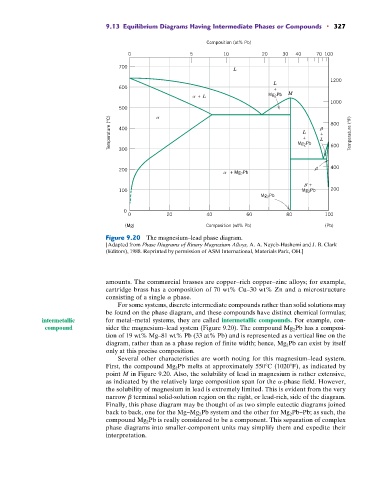
Figure 9.20 The magnesium–lead phase diagram.
[Adapted from Phase Diagrams of Binary Magnesium Alloys, A. A. Nayeb-Hashemi and J. B. Clark
(Editors), 1988. Reprinted by permission of ASM International, Materials Park, OH.]
amounts. The commercial brasses are copper–rich copper–zinc alloys; for example,
cartridge brass has a composition of 70 wt% Cu–30 wt% Zn and a microstructure
consisting of a single a phase.
For some systems, discrete intermediate compounds rather than solid solutions may
be found on the phase diagram, and these compounds have distinct chemical formulas;
intermetallic for metal–metal systems, they are called intermetallic compounds. For example, con-
compound sider the magnesium–lead system (Figure 9.20). The compound Mg 2 Pb has a composi-
tion of 19 wt% Mg–81 wt% Pb (33 at% Pb) and is represented as a vertical line on the
diagram, rather than as a phase region of finite width; hence, Mg 2 Pb can exist by itself
only at this precise composition.
Several other characteristics are worth noting for this magnesium–lead system.
First, the compound Mg 2 Pb melts at approximately 550 C (1020 F), as indicated by
point M in Figure 9.20. Also, the solubility of lead in magnesium is rather extensive,
as indicated by the relatively large composition span for the a-phase field. However,
the solubility of magnesium in lead is extremely limited. This is evident from the very
narrow b terminal solid-solution region on the right, or lead-rich, side of the diagram.
Finally, this phase diagram may be thought of as two simple eutectic diagrams joined
back to back, one for the Mg–Mg 2 Pb system and the other for Mg 2 Pb–Pb; as such, the
compound Mg 2 Pb is really considered to be a component. This separation of complex
phase diagrams into smaller-component units may simplify them and expedite their
interpretation.