Page 360 - Materials Science and Engineering An Introduction
P. 360
332 • Chapter 9 / Phase Diagrams
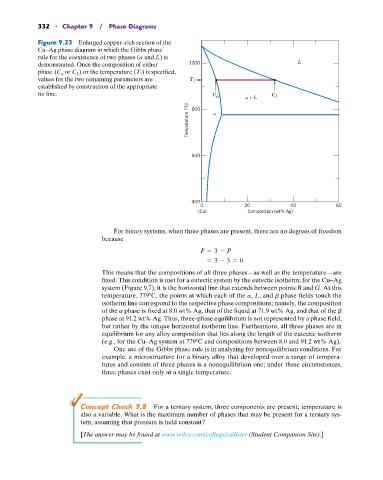
Figure 9.23 Enlarged copper–rich section of the
Cu–Ag phase diagram in which the Gibbs phase
rule for the coexistence of two phases (a and L) is
demonstrated. Once the composition of either 1000 L
phase (C a or C L ) or the temperature (T 1 ) is specified,
values for the two remaining parameters are T 1
established by construction of the appropriate
tie line. C C
+ L L
Temperature (°C) 800
600
400
0 20 40 60
(Cu) Composition (wt% Ag)
For binary systems, when three phases are present, there are no degrees of freedom
because
F = 3 - P
= 3 - 3 = 0
This means that the compositions of all three phases—as well as the temperature—are
fixed. This condition is met for a eutectic system by the eutectic isotherm; for the Cu–Ag
system (Figure 9.7), it is the horizontal line that extends between points B and G. At this
temperature, 779 C, the points at which each of the a, L, and b phase fields touch the
isotherm line correspond to the respective phase compositions; namely, the composition
of the a phase is fixed at 8.0 wt% Ag, that of the liquid at 71.9 wt% Ag, and that of the b
phase at 91.2 wt% Ag. Thus, three-phase equilibrium is not represented by a phase field,
but rather by the unique horizontal isotherm line. Furthermore, all three phases are in
equilibrium for any alloy composition that lies along the length of the eutectic isotherm
(e.g., for the Cu–Ag system at 779 C and compositions between 8.0 and 91.2 wt% Ag).
One use of the Gibbs phase rule is in analyzing for nonequilibrium conditions. For
example, a microstructure for a binary alloy that developed over a range of tempera-
tures and consists of three phases is a nonequilibrium one; under these circumstances,
three phases exist only at a single temperature.
Concept Check 9.8 For a ternary system, three components are present; temperature is
also a variable. What is the maximum number of phases that may be present for a ternary sys-
tem, assuming that pressure is held constant?
[The answer may be found at www.wiley.com/college/callister (Student Companion Site).]