Page 209 - Modeling of Chemical Kinetics and Reactor Design
P. 209
Reaction Rate Expression 179
4− 3−
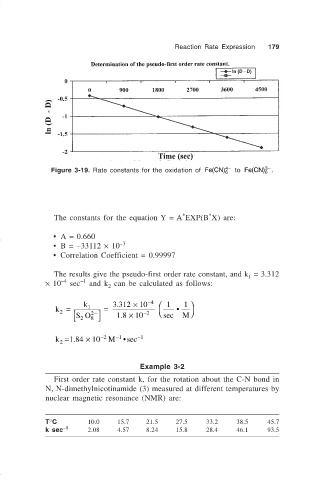
Figure 3-19. Rate constants for the oxidation of Fe CN( ) 6 to Fe CN( ) 6 .
*
*
The constants for the equation Y = A EXP(B X) are:
• A = 0.660
• B = –33112 × 10 –3
• Correlation Coefficient = 0.99997
The results give the pseudo-first order rate constant, and k = 3.312
1
–4
–1
× 10 sec and k can be calculated as follows:
2
.
k = k 1 = 3 312 × 10 − 4 1 • 1
2 [
2−
2 SO ] 18 × 10 − 2 sec M
.
8
−
k = 184 × 10 − 2 M •sec − 1
1
.
2
Example 3-2
First order rate constant k, for the rotation about the C-N bond in
N, N-dimethylnicotinamide (3) measured at different temperatures by
nuclear magnetic resonance (NMR) are:
T°C 10.0 15.7 21.5 27.5 33.2 38.5 45.7
k sec –1 2.08 4.57 8.24 15.8 28.4 46.1 93.5