Page 212 - Modeling of Chemical Kinetics and Reactor Design
P. 212
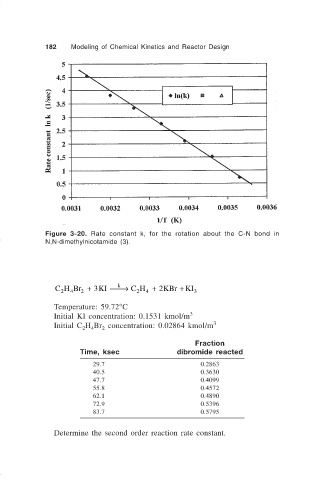
182 Modeling of Chemical Kinetics and Reactor Design
Figure 3-20. Rate constant k, for the rotation about the C-N bond in
N,N-dimethylnicotamide (3).
C H Br + 3 KI → C H + 2 KBr + KI 3
k
4
2
2
4
2
Temperature: 59.72°C
Initial KI concentration: 0.1531 kmol/m 3
Initial C H Br concentration: 0.02864 kmol/m 3
2
4
2
Fraction
Time, ksec dibromide reacted
29.7 0.2863
40.5 0.3630
47.7 0.4099
55.8 0.4572
62.1 0.4890
72.9 0.5396
83.7 0.5795
Determine the second order reaction rate constant.