Page 210 - Modeling of Chemical Kinetics and Reactor Design
P. 210
180 Modeling of Chemical Kinetics and Reactor Design
Determine the activation energy, E, and the pre-exponential factor,
k , for the rotation.
O
Solution
The activation energy E is a measure of the temperature sensitivity
of the rate constant. A high E corresponds to a rate constant that
increases rapidly with temperature. From the Arrhenius equation
−
E
k = k exp RT (3-251)
O
T
where k = rate constant at known temperature
T
k = pre-exponential factor
O
E = activation energy
R = gas constant (8.314 J/mol • K)
T = absolute temperature (K = 273.15 + °C)
It is possible to determine the activation energy E and the pre-
exponential factor k .
0
Linearizing Equation 3-251 gives
ln k = ln k − E
T O (3-252)
RT
By plotting ln k against 1/T, the slope equal to –E/R is obtained
T
and the intercept equal to ln k . From these known constants, activa-
O
tion energy E and the pre-exponential factor k are determined.
O
Equation 3-251 is of the form
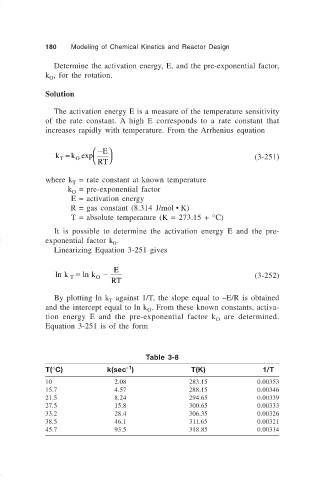
Table 3-8
–1
T(°C) k(sec ) T(K) 1/T
10 2.08 283.15 0.00353
15.7 4.57 288.15 0.00346
21.5 8.24 294.65 0.00339
27.5 15.8 300.65 0.00333
33.2 28.4 306.35 0.00326
38.5 46.1 311.65 0.00321
45.7 93.5 318.85 0.00314