Page 276 - Modeling of Chemical Kinetics and Reactor Design
P. 276
246 Modeling of Chemical Kinetics and Reactor Design
beginning. Also, the reaction may follow different reaction paths as
the catalyst decays, thereby varying the selectivity of a particular
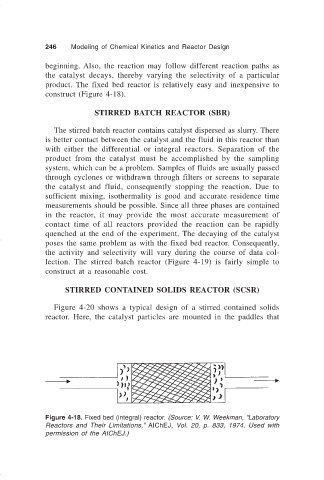
product. The fixed bed reactor is relatively easy and inexpensive to
construct (Figure 4-18).
STIRRED BATCH REACTOR (SBR)
The stirred batch reactor contains catalyst dispersed as slurry. There
is better contact between the catalyst and the fluid in this reactor than
with either the differential or integral reactors. Separation of the
product from the catalyst must be accomplished by the sampling
system, which can be a problem. Samples of fluids are usually passed
through cyclones or withdrawn through filters or screens to separate
the catalyst and fluid, consequently stopping the reaction. Due to
sufficient mixing, isothermality is good and accurate residence time
measurements should be possible. Since all three phases are contained
in the reactor, it may provide the most accurate measurement of
contact time of all reactors provided the reaction can be rapidly
quenched at the end of the experiment. The decaying of the catalyst
poses the same problem as with the fixed bed reactor. Consequently,
the activity and selectivity will vary during the course of data col-
lection. The stirred batch reactor (Figure 4-19) is fairly simple to
construct at a reasonable cost.
STIRRED CONTAINED SOLIDS REACTOR (SCSR)
Figure 4-20 shows a typical design of a stirred contained solids
reactor. Here, the catalyst particles are mounted in the paddles that
Figure 4-18. Fixed bed (integral) reactor. (Source: V. W. Weekman, “Laboratory
Reactors and Their Limitations,” AIChEJ, Vol. 20, p. 833, 1974. Used with
permission of the AIChEJ.)