Page 275 - Modeling of Chemical Kinetics and Reactor Design
P. 275
Industrial and Laboratory Reactors 245
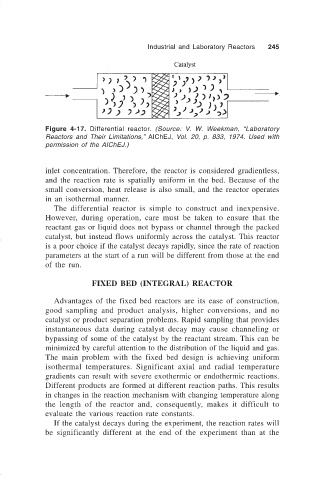
Figure 4-17. Differential reactor. (Source: V. W. Weekman, “Laboratory
Reactors and Their Limitations,” AIChEJ, Vol. 20, p. 833, 1974. Used with
permission of the AIChEJ.)
inlet concentration. Therefore, the reactor is considered gradientless,
and the reaction rate is spatially uniform in the bed. Because of the
small conversion, heat release is also small, and the reactor operates
in an isothermal manner.
The differential reactor is simple to construct and inexpensive.
However, during operation, care must be taken to ensure that the
reactant gas or liquid does not bypass or channel through the packed
catalyst, but instead flows uniformly across the catalyst. This reactor
is a poor choice if the catalyst decays rapidly, since the rate of reaction
parameters at the start of a run will be different from those at the end
of the run.
FIXED BED (INTEGRAL) REACTOR
Advantages of the fixed bed reactors are its ease of construction,
good sampling and product analysis, higher conversions, and no
catalyst or product separation problems. Rapid sampling that provides
instantaneous data during catalyst decay may cause channeling or
bypassing of some of the catalyst by the reactant stream. This can be
minimized by careful attention to the distribution of the liquid and gas.
The main problem with the fixed bed design is achieving uniform
isothermal temperatures. Significant axial and radial temperature
gradients can result with severe exothermic or endothermic reactions.
Different products are formed at different reaction paths. This results
in changes in the reaction mechanism with changing temperature along
the length of the reactor and, consequently, makes it difficult to
evaluate the various reaction rate constants.
If the catalyst decays during the experiment, the reaction rates will
be significantly different at the end of the experiment than at the