Page 278 - Modeling of Chemical Kinetics and Reactor Design
P. 278
248 Modeling of Chemical Kinetics and Reactor Design
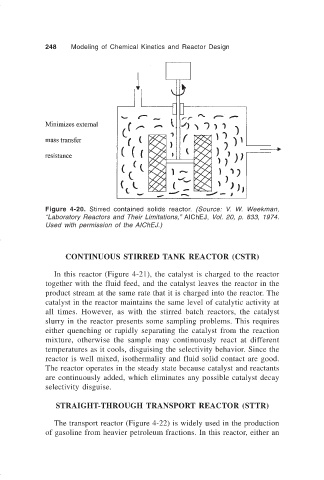
Figure 4-20. Stirred contained solids reactor. (Source: V. W. Weekman,
“Laboratory Reactors and Their Limitations,” AIChEJ, Vol. 20, p. 833, 1974.
Used with permission of the AIChEJ.)
CONTINUOUS STIRRED TANK REACTOR (CSTR)
In this reactor (Figure 4-21), the catalyst is charged to the reactor
together with the fluid feed, and the catalyst leaves the reactor in the
product stream at the same rate that it is charged into the reactor. The
catalyst in the reactor maintains the same level of catalytic activity at
all times. However, as with the stirred batch reactors, the catalyst
slurry in the reactor presents some sampling problems. This requires
either quenching or rapidly separating the catalyst from the reaction
mixture, otherwise the sample may continuously react at different
temperatures as it cools, disguising the selectivity behavior. Since the
reactor is well mixed, isothermality and fluid solid contact are good.
The reactor operates in the steady state because catalyst and reactants
are continuously added, which eliminates any possible catalyst decay
selectivity disguise.
STRAIGHT-THROUGH TRANSPORT REACTOR (STTR)
The transport reactor (Figure 4-22) is widely used in the production
of gasoline from heavier petroleum fractions. In this reactor, either an