Page 40 - Modelling in Transport Phenomena A Conceptual Approach
P. 40
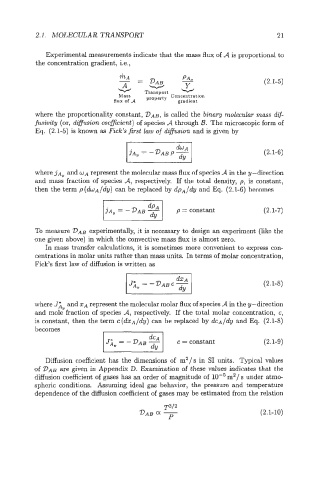
2.1. MOLECULAR TRANSPORT 21
Experimental measurements indicate that the mass flux of A is proportional to
the concentration gradient, i.e.,
-- - (2.1-5)
hA
PA,
- DAB
A w Y
Transport
Mass property Concentration
flux of A gradient
where the proportionality constant, DAB, is called the binary molecular mass dif-
fusivity (or, difusion coeficient) of species A through B. The microscopic form of
Eq. (2.1-5) is known as Fick’s first law of diffusion and is given by
dY I
~A~=-DABP-- (2.1-6)
where jAs and WA represent the molecular mass flux of species A in the y-direction
and mass fraction of species A, respectively. If the total density, p, is constant,
then the term p (dwA/dy) can be replaced by dpA/dy and Eq. (2.1-6) becomes
p = constant (2.1-7)
To measure VAB experimentally, it is necessary to design an experiment (like the
one given above) in which the convective mass flux is almost zero.
In mass transfer calculations, it is sometimes more convenient to express con-
centrations in molar units rather than mass units. In terms of molar concentration,
Fick’s first law of diffusion is written as
(2.1-8)
where J:s and ZA represent the molecular molar flux of species A in the y-direction
and mole fraction of species A, respectively. If the total molar concentration, c,
is constant, then the term c(dz~/dy) can be replaced by dcA/dy and Eq. (2.1-8)
- 1 c= constant (2.1-9)
becomes
Diffusion coefficient has the dimensions of m2/s in SI units. Typical values
of DAB are given in Appendix D. Examination of these values indicates that the
diffusion coefficient of gases has an order of magnitude of m2/ s under atme
spheric conditions. Assuming ideal gas behavior, the pressure and temperature
dependence of the diffusion coefficient of gases may be estimated from the relation
(2.1-10)