Page 569 - Modelling in Transport Phenomena A Conceptual Approach
P. 569
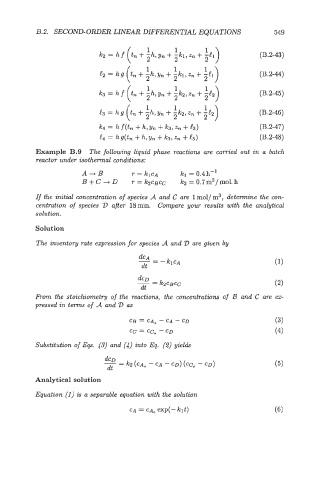
B.2. SECOND-ORDER LINEAR DLFFEmNTIAL EQUATIONS 549
(B.2-43)
(B.2-45)
(B .2-46)
(B.2-47)
(B .2-48)
Example B.9 The following liquid phase reactions are carried out in a batch
reactor under isothermal conditions:
A4B T = klCA kl = 0.4 h-l
B + C D r = k2C~Cc b = 0.7m3/mol. h
-+
If the initial concentration of species A and C are 1 mol/ m3, determine the wn-
centration of species 2) after 18min. Compare your results with the analytical
solution.
Solution
The inventory rate expression for species A and D are given by
From the stoichiometry of the reactions, the concentrations of L3 and C are ex-
pressed in terms of A and 2) as
CB = CA, - CA - CD (3)
cc = CC, - CD (4)
Substitution of Eqs. (3) and (4) into Eq. (2) yields
Analytical solution
Equation (1) is a separable equation with the solution
CA = CA, exp(- klt) (6)