Page 211 - Book Hosokawa Nanoparticle Technology Handbook
P. 211
4.2 NANOPARTICLES ARRANGED STRUCTURES FUNDAMENTALS
[9] K. Kjaergaard, J.K. Sørensen, M.A. Schembri, [20] C. Mao, C.E. Flynn, A. Hayhurst, R. Sweeney, J. Qi,
P. Klemm: Appl. Environ. Microbiol., 66, 10–14 (2000). G. Georgiou, B. Iverson, A.M. Belcher: Proc. Natl.
[10] M. Umetsu, M. Mizuta, K. Tsumoto, S. Ohara, Acad. Sci. USA., 100, 6946–6951 (2003).
S. Takami, H. Watanabe, I. Kumagai, T. Adschiri: [21] C. Mao, D.J. Solis, B.D. Reiss, S.T. Kottmann,
Adv. Mater., 17, 2571–2575 (2005). R.Y. Sweeney, A. Hayhurst, G. Georgiou, B. Iverson,
[11] S. Nygaard, R. Wendelbo, S. Brown: Adv. Mater., 14, A.M. Belcher: Science, 303, 213–217 (2004).
1853–1856 (2002).
[12] D.J.H. Gaskin, K. Starck, E.N. Vulfson: Biotech. Lett.,
4.2.3 Preparation of ceramic films by liquid-phase
22, 1211–1216 (2000).
processing: Electrophoresis
[13] R.R. Naik, S.J. Stringer, G. Agarwal, S.E. Jones,
M.O. Stone MO: Nat. Mater., 1, 169–172 (2002).
Colloidal processing, which is composed of the disper-
[14] R.R. Naik, L.L. Brott, S.J. Clarson, M.O. Stone: sion of ceramic powders in liquid media followed by
J. Nanosci. Nanotechnol., 2, 95–100 (2002). consolidation, is superior to conventional dry process-
[15] S.W. Lee, C.B. Mao, C.E. Flynn, A.M. Belcher: ing in the control of density and microstructure of
Science, 296, 892–895 (2002). green and sintered bodies. Electrophoretic deposition
[16] K. Sano, K. Shiba: J. Am. Chem. Soc., 125, (EPD) is one of the most promising colloidal processes
14234–14235 (2003). wherein ceramic bodies are directly shaped from a
stable colloidal suspension by a dc electric field. Elec-
[17] D. Kase, J.L. Kulp, M. Yudasaka, J.S. Evans, S. Iijima,
trically conductive metals or graphite are often used
K. Shiba: Langmiur, 20, 8939–8941 (2004).
both as an electrode and a substrate, however a non-
[18] S. Wang, E.S. Humphreys, S.Y. Chung, D.F. Delduco,
conductive porous material, which is placed in front of
S.R. Lustig, H. Wang, K.N. Parker, N.W. Rizzo,
an electrode non-contactedly, is also possible to use as
S. Subramoney, Y.M. Chiang, A. Jagota: Nat. Mater., a substrate. EPD is a combination of two processes:
2, 196–200 (2003). electrophoresis of charged particles in a suspension
[19] M. Sarikaya, C. Tamerler, A.K. Jen, K. Schulten, and deposition on a substrate. The mechanism of
F. Baneyx: Nat. Mater., 2, 577–585 (2003). deposition is explained based on the DLVO theory; the
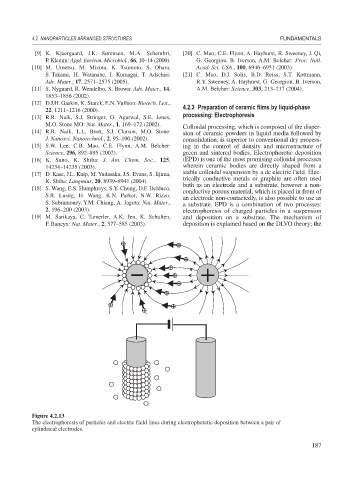
Figure 4.2.13
The electrophoresis of particles and electric field lines during electrophoretic deposition between a pair of
cylindrical electrodes.
187