Page 212 - Book Hosokawa Nanoparticle Technology Handbook
P. 212
FUNDAMENTALS CH. 4 CONTROL OF NANOSTRUCTURE OF MATERIALS
deposition is due to the coagulation by the van der between the particles is weak; it may cause slip-off of
Waals attraction accompanied by the drop of the repul- the deposits when the applied dc is turned off.
sive potential at an electrode. Figure 4.2.13 shows the The weight of deposit, W(g), during electrophoretic
electrophoresis of particles and electric field lines dur- deposition is estimated based on the mass balance law
ing electrophoretic deposition between a pair of cylin- of Hamaker [1];
drical electrodes. The surface-charged particles do not
take the shortest straight route but move along the elec- dW
tric field lines to an oppositely charged electrode in a dt fCES (4.2.4)
liquid. Therefore, the particles deposit even on the back
of a substrate. This characteristic enables the EPD to where t is the deposition time (s), the electrophoretic
make uniform surface coating on a substrate with mobility (m /Vs), E the electric field strength (V/m), C
2
uneven surface and complex shape. 3
the solid loading (g/m ), S the surface area of the elec-
2
trode (m ), and f the sticking probability of the particles
1. Kinetics of EPD processing
that arrived at the substrate (0 f 1). The electric
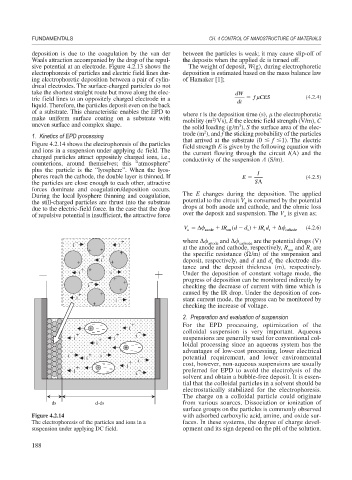
Figure 4.2.14 shows the electrophoresis of the particles field strength E is given by the following equation with
and ions in a suspension under applying dc field. The the current flowing through the circuit I(A) and the
charged particles attract oppositely charged ions, i.e., conductivity of the suspension (S/m).
counterions, around themselves; this “atmosphere”
plus the particle is the “lyosphere”. When the lyos- I
pheres reach the cathode, the double layer is thinned. If E (4.2.5)
the particles are close enough to each other, attractive S
forces dominate and coagulation/deposition occurs.
During the local lyosphere thinning and coagulation, The E changes during the deposition. The applied
the still-charged particles are thrust into the substrate potential to the circuit V is consumed by the potential
a
due to the electric-field force. In the case that the drop drops at both anode and cathode, and the ohmic loss
of repulsive potential is insufficient, the attractive force over the deposit and suspension. The V is given as;
a
V Δ anode IR ( d d IR d cathode (4.2.6)
)
s
s
s
sus
a
where and are the potential drops (V)
i i anode cathode
at the anode and cathode, respectively, R sus and R are
s
the specific resistance ( /m) of the suspension and
i deposit, respectively, and d and d the electrode dis-
s
tance and the deposit thickness (m), respectively.
i i Under the deposition of constant voltage mode, the
progress of deposition can be monitored indirectly by
i checking the decrease of current with time which is
caused by the IR drop. Under the deposition of con-
i
i stant current mode, the progress can be monitored by
checking the increase of voltage.
2. Preparation and evaluation of suspension
For the EPD processing, optimization of the
i
colloidal suspension is very important. Aqueous
i
i i suspensions are generally used for conventional col-
i loidal processing since an aqueous system has the
advantages of low-cost processing, lower electrical
i i
i potential requirement, and lower environmental
i
i cost, however, non-aqueous suspensions are usually
i preferred for EPD to avoid the electrolysis of the
i
solvent and obtain a bubble-free deposit. It is essen-
tial that the colloidal particles in a solvent should be
electrostatically stabilized for the electrophoresis.
The charge on a colloidal particle could originate
ds d-ds from various sources. Dissociation or ionization of
surface groups on the particles is commonly observed
Figure 4.2.14 with adsorbed carboxylic acid, amine, and oxide sur-
The electrophoresis of the particles and ions in a faces. In these systems, the degree of charge devel-
suspension under applying DC field. opment and its sign depend on the pH of the solution.
188