Page 213 - Book Hosokawa Nanoparticle Technology Handbook
P. 213
4.2 NANOPARTICLES ARRANGED STRUCTURES FUNDAMENTALS
For example, surfaces of oxide particles tend to 10 40
coordinate water molecules to form amphoteric
hydroxyl groups that can charge positively or nega- 9 35
tively depending on pH of liquid media: 8 30
7
(M OH ) H (M OH 2 ) (at low pH ) (4.2.7) 6 25
(M OH ) OH (M O ) H O (at high pH ) (4.2.8) pH op 5 20 Conductivity (µS/cm)
2
15
4
where H and /or OH behaves as potential- 3 10
determining ions. Addition of acid or base and/or 5
polyelectrolytes helps to enhance the surface charge 2
potentials of particles, however too much addition of 1 0
them can be countereffective on the stability of a 0 0.1 0.2 0.3 0.4
suspension since non-adsorbed free ions causes the Amount of SQA (wt%)
double-layer compaction.
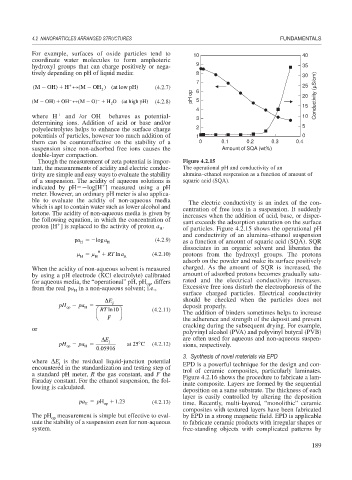
Though the measurement of zeta potential is impor- Figure 4.2.15
tant, the measurements of acidity and electric conduc- The operational pH and conductivity of an
tivity are simple and easy ways to evaluate the stability alumina–ethanol suspension as a function of amount of
of a suspension. The acidity of aqueous solutions is squaric acid (SQA).
indicated by pH log[H ] measured using a pH
meter. However, an ordinary pH meter is also applica-
ble to evaluate the acidity of non-aqueous media The electric conductivity is an index of the con-
which is apt to contain water such as lower alcohol and centration of free ions in a suspension. It suddenly
ketone. The acidity of non-aqueous media is given by increases when the addition of acid, base, or disper-
the following equation, in which the concentration of sant exceeds the adsorption saturation on the surface
proton [H ] is replaced to the activity of proton a . of particles. Figure 4.2.15 shows the operational pH
H
and conductivity of an alumina–ethanol suspension
pa log a H (4.2.9) as a function of amount of squaric acid (SQA). SQR
H
dissociates in an organic solvent and liberates the
H 0 RT lna H (4.2.10) protons from the hydroxyl groups. The protons
H
adsorb on the powder and make its surface positively
When the acidity of non-aqueous solvent is measured charged. As the amount of SQR is increased, the
by using a pH electrode (KCl electrolyte) calibrated amount of adsorbed protons becomes gradually satu-
for aqueous media, the “operational” pH, pH , differs rated and the electrical conductivity increases.
op
from the real pa in a non-aqueous solvent; i.e., Excessive free ions disturb the electrophoresis of the
H
surface charged particles. Electrical conductivity
E j should be checked when the particles does not
pH pa deposit properly.
op
⎛ RT ln10⎞ (4.2.11)
H
⎜ ⎝ F ⎟ ⎠ The addition of binders sometimes helps to increase
the adherence and strength of the deposit and prevent
cracking during the subsequent drying. For example,
or
polyvinyl alcohol (PVA) and polyvinyl butyral (PVB)
E j are often used for aqueous and non-aqueous suspen-
pH pa at 25 C (4.2.12) sions, respectively.
op
H
0 05916
.
3. Synthesis of novel materials via EPD
where E is the residual liquid-junction potential EPD is a powerful technique for the design and con-
j
encountered in the standardization and testing step of trol of ceramic composites, particularly laminates.
a standard pH meter, R the gas constant, and F the Figure 4.2.16 shows the procedure to fabricate a lam-
Faraday constant. For the ethanol suspension, the fol- inate composite. Layers are formed by the sequential
lowing is calculated.
deposition on a same substrate. The thickness of each
layer is easily controlled by altering the deposition
pa pH 123 (4.2.13) time. Recently, multi-layered, “monolithic” ceramic
.
op
H
composites with textured layers have been fabricated
The pH measurement is simple but effective to eval- by EPD in a strong magnetic field. EPD is applicable
op
uate the stability of a suspension even for non-aqueous to fabricate ceramic products with irregular shapes or
system. free-standing objects with complicated patterns by
189