Page 454 - Book Hosokawa Nanoparticle Technology Handbook
P. 454
APPLICATIONS 2 GENERATION OF METAL NANOPARTICLES
for advanced printed boards with thin conductive [5] Arakawa Chemical Industries Ltd., Patent, WO01-
layers and flat interfaces. 05862, EP1123944, CN1318077T, TW483907,
US6506868.
[6] H. Goda: J. Jpn. Soc. Colour Mater., 77 (2), 69 (2004).
References
[7] H. Goda: Polym. Prepr. Jpn., 50, 2688 (2001).
[1] B.C. Novak: Adv. Mater., 5 (6), 422 (1993). [8] H. Goda, C.W. Frank: Chem. Mater., 13 (7), 2783
[2] Y. Chujo, T. Saegusa: Adv. Ploym. Sci., 100, 11 (1992). (2001).
[3] G.L. Wilkes, B. Orler and H.H. Haung: Polym. Prep., [9] H. Goda: Kobunshi Ronbunshu, 59, 596 (2002).
26 (2), 300 (1985). [10] H. Goda, M. Mesaki: Polym. Prepr. Jpn., 51, 2245
[4] S. Yamazaki: Polyurethane–Silica hybrid, Technical (2002).
Report of National Institute for Material Science, [11] H. Goda, T. Fujiwara: Expected Mater. Future, 3, 34
Japan, 4, 41 (1996). (2003).
APPLICATION 2
2 GENERATION OF METAL NANOPARTICLES USING REACTIVE PLASMA
ARC EVAPORATION
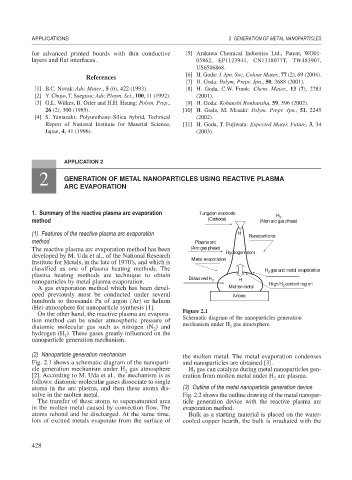
1. Summary of the reactive plasma arc evaporation Tungsten electrode H
2
method (Cathode) (Non arc gas phase)
(1) Features of the reactive plasma arc evaporation H Nanoparticles
method Plasma arc
The reactive plasma arc evaporation method has been (Arc gas phase) Hydrogen atom
developed by M. Uda et al., of the National Research Metal evaporation
Institute for Metals, in the late of 1970’s, and which is
classified as one of plasma heating methods. The H gas and metal evaporation
2
plasma heating methods are technique to obtain Dissolved H
nanoparticles by metal plasma evaporation. 2 H High H content region
A gas evaporation method which has been devel- Molten metal 2
oped previously must be conducted under several Anode
hundreds to thousands Pa of argon (Ar) or helium
(He) atmosphere for nanoparticle synthesis [1]. Figure 2.1
On the other hand, the reactive plasma arc evapora-
tion method can be under atmospheric pressure of Schematic diagram of the nanoparticles generation
mechanism under H gas atmosphere.
diatomic molecular gas such as nitrogen (N ) and 2
2
hydrogen (H ). These gases greatly influenced on the
2
nanoparticle generation mechanism.
(2) Nanoparticle generation mechanism the molten metal. The metal evaporation condenses
Fig. 2.1 shows a schematic diagram of the nanoparti- and nanoparticles are obtained [3].
cle generation mechanism under H gas atmosphere H gas can catalyze during metal nanoparticles gen-
2
2
[2]. According to M. Uda et al., the mechanism is as eration from molten metal under H arc plasma.
2
follows: diatomic molecular gases dissociate to single
atoms in the arc plasma, and then these atoms dis- (3) Outline of the metal nanoparticle generation device
solve in the molten metal. Fig. 2.2 shows the outline drawing of the metal nanopar-
The transfer of these atoms to supersaturated area ticle generation device with the reactive plasma arc
in the molten metal caused by convection flow. The evaporation method.
atoms rebond and be discharged. At the same time, Bulk as a starting material is placed on the water-
lots of excited metals evaporate from the surface of cooled copper hearth, the bulk is irradiated with the
428