Page 459 - Book Hosokawa Nanoparticle Technology Handbook
P. 459
3 SENSING BASED ON LOCALIZED SURFACE PLASMON RESONANCE APPLICATIONS
analyte
increased thickness
ligand (receptor)
Substrate or surface of nanoparticles
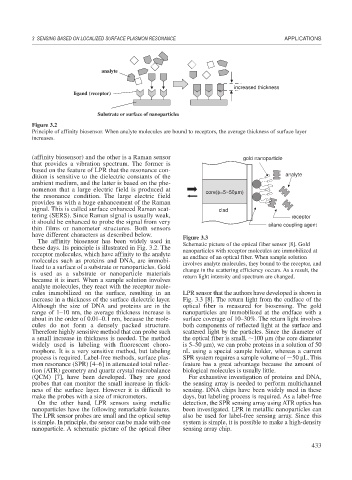
Figure 3.2
Principle of affinity biosensor. When analyte molecules are bound to receptors, the average thickness of surface layer
increases.
(affinity biosensor) and the other is a Raman sensor gold nanoparticle
that provides a vibration spectrum. The former is
based on the feature of LPR that the resonance con-
dition is sensitive to the dielectric constants of the analyte
ambient medium, and the latter is based on the phe-
nomenon that a large electric field is produced at core(φ=5~50μm)
the resonance condition. The large electric field
provides us with a huge enhancement of the Raman
signal. This is called surface enhanced Raman scat- clad
tering (SERS). Since Raman signal is usually weak, receptor
it should be enhanced to probe the signal from very silane coupling agent
thin films or nanometer structures. Both sensors
have different characters as described below. Figure 3.3
The affinity biosensor has been widely used in
these days. Its principle is illustrated in Fig. 3.2. The Schematic picture of the optical fiber sensor [8]. Gold
nanoparticles with receptor molecules are immobilized at
receptor molecules, which have affinity to the analyte an endface of an optical fiber. When sample solution
molecules such as proteins and DNA, are immobi- involves analyte molecules, they bound to the receptor, and
lized to a surface of a substrate or nanoparticles. Gold change in the scattering efficiency occurs. As a result, the
is used as a substrate or nanoparticle materials return light intensity and spectrum are changed.
because it is inert. When a sample solution involves
analyte molecules, they react with the receptor mole-
cules immobilized on the surface, resulting in an LPR sensor that the authors have developed is shown in
increase in a thickness of the surface dielectric layer. Fig. 3.3 [8]. The return light from the endface of the
Although the size of DNA and proteins are in the optical fiber is measured for biosensing. The gold
range of 1–10 nm, the average thickness increase is nanoparticles are immobilized at the endface with a
about in the order of 0.01–0.1 nm, because the mole- surface coverage of 10–30%. The return light involves
cules do not form a densely packed structure. both components of reflected light at the surface and
Therefore highly sensitive method that can probe such scattered light by the particles. Since the diameter of
a small increase in thickness is needed. The method the optical fiber is small, 100 m (the core diameter
widely used is labeling with fluorescent choro- is 5–50 m), we can probe proteins in a solution of 50
mophore. It is a very sensitive method, but labeling nL using a special sample holder, whereas a current
process is required. Label-free methods, surface plas- SPR system requires a sample volume of 50 L. This
mon resonance (SPR) [4–6] in attenuated total reflec- feature has a great advantage because the amount of
tion (ATR) geometry and quartz crystal microbalance biological molecules is usually little.
(QCM) [7], have been developed. They are good For exhaustive investigation of proteins and DNA,
probes that can monitor the small increase in thick- the sensing array is needed to perform multichannel
ness of the surface layer. However it is difficult to sensing. DNA chips have been widely used in these
make the probes with a size of micrometers. days, but labeling process is required. As a label-free
On the other hand, LPR sensors using metallic detection, the SPR sensing array using ATR optics has
nanoparticles have the following remarkable features. been investigated. LPR in metallic nanoparticles can
The LPR sensor probes are small and the optical setup also be used for label-free sensing array. Since this
is simple. In principle, the sensor can be made with one system is simple, it is possible to make a high-density
nanoparticle. A schematic picture of the optical fiber sensing array chip.
433