Page 579 - Book Hosokawa Nanoparticle Technology Handbook
P. 579
31 DEVELOPMENT OF EXHAUST CATALYST APPLICATIONS
Stoichiometric In fuel-rich atmosphere, partial Ce ions are trivalent.
fuel rich fuel lean However, molar fraction of trivalent Ce ions is only a
100 small amount in the total Ce ions. The molar fractions
of trivalent Ce ions of CeO , X/2, at 500 C in fuel-rich
2
HC or CO
NOx atmosphere, after durability test at 900 and 1200 C
were 0.01 and 0.002, respectively, in the study by
present authors [13]. The reduction in the value of X/2
wide at 500 C is ascribed by the fact that only the oxygen
Conversion (%) 50 operating Therefore, oxygen-storage materials of early years
ions on the surface can contribute OSC [14].
narrow
have a weak point that OSC decreases with the
decreasing specific surface area of CeO after such a
2
window
high-temperature durability test. BaO and La O had
3
2
an effect in preventing sintering and maintaining spe-
cific area of CeO ; however, it was not enough [10].
2
3. Improvement of OSC of catalyst
0 14.6 Two types of new technological developments are
Air/Fuel ratio (wt./wt.) introduced concerning the nanolevel particles
achieved in the three-way catalyst system in recent
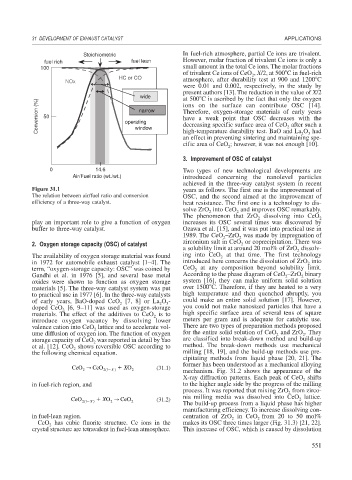
Figure 31.1 years as follows. The first one is the improvement of
The relation between air/fuel ratio and conversion OSC, and the second aimed at the improvement of
efficiency of a three-way catalyst. heat resistance. The first one is a technology to dis-
solve ZrO into CeO and improves OSC remarkably.
2
2
The phenomenon that ZrO dissolving into CeO 2
2
play an important role to give a function of oxygen increases its OSC several times was discovered by
buffer to three-way catalyst. Ozawa et al. [15], and it was put into practical use in
1989. The CeO –ZrO was made by impregnation of
2
2
zirconium salt in CeO or coprecipitation. There was
2. Oxygen storage capacity (OSC) of catalyst 2
a solubility limit at around 20 mol% of ZrO dissolv-
2
The availability of oxygen storage material was found ing into CeO at that time. The first technology
2
in 1972 for automobile exhaust catalyst [1–4]. The introduced here concerns the dissolution of ZrO into
2
term, “oxygen-storage capacity: OSC” was coined by CeO at any composition beyond solubility limit.
2
Gandhi et al. in 1976 [5], and several base metal According to the phase diagram of CeO –ZrO binary
2
2
oxides were shown to function as oxygen storage system [16], they can make uniform solid solution
materials [5]. The three-way catalyst system was put over 1500 C. Therefore, if they are heated to a very
to practical use in 1977 [6]. In the three-way catalysts high temperature and then quenched abruptly, you
of early years, BaO-doped CeO [7, 8] or La O - could make an entire solid solution [17]. However,
3
2
2
doped CeO [6, 9–11] was used as oxygen-storage you could not make nanosized particles that have a
2
materials. The effect of the additives to CeO is to high specific surface area of several tens of square
2
introduce oxygen vacancy by dissolving lower meters per gram and is adequate for catalytic use.
valence cation into CeO lattice and to accelerate vol- There are two types of preparation methods proposed
2
ume diffusion of oxygen ion. The function of oxygen for the entire solid solution of CeO and ZrO . They
2
2
storage capacity of CeO was reported in detail by Yao are classified into break-down method and build-up
2
et al. [12]. CeO shows reversible OSC according to method. The break-down methods use mechanical
2
the following chemical equation. milling [18, 19], and the build-up methods use pre-
cipitating methods from liquid phase [20, 21]. The
CeO CeO XO (31.1) former has been understood as a mechanical alloying
(
2 2 1 X ) 2 mechanism. Fig. 31.2 shows the appearance of the
X-ray diffraction patterns. Each peak of CeO shifts
2
in fuel-rich region, and to the higher angle side by the progress of the milling
process. It was reported that mixing ZrO from zirco-
2
CeO X O CeO (31.2) nia milling media was dissolved into CeO lattice.
2
21 X ) 2 2
(
The build-up process from a liquid phase has higher
manufacturing efficiency. To increase dissolving con-
in fuel-lean region. centration of ZrO in CeO from 20 to 50 mol%
2
2
CeO has cubic fluorite structure. Ce ions in the makes its OSC three times larger (Fig. 31.3) [21, 22].
2
crystal structure are tetravalent in fuel-lean atmosphere. This increase of OSC, which is caused by dissolution
551