Page 580 - Book Hosokawa Nanoparticle Technology Handbook
P. 580
APPLICATIONS 31 DEVELOPMENT OF EXHAUST CATALYST
°
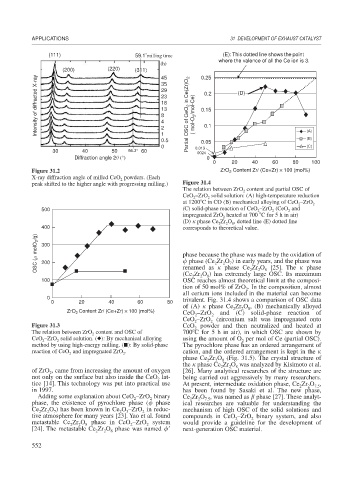
(111) 59.1 milling time (E): This dotted line shows the point
where the valence of all the Ce ion is 3.
(h)
(200) (220) (311) 45 0.25
Intensity of diffracted X-ray 29 Partial OSC of CeO 2 in Ce(Zr)O 2 ( mol-O 2 /mol-Ce) 0.15 (D)
35
0.2
23
18
13
8
4
0.1
2
1
(B)
0.5 0.05 (A)
0 0.013 (C)
30 40 50 56.3° 60 0024
Diffraction angle 2 (°) 0
0 20 40 60 80 100
Figure 31.2 ZrO Content Zr/ (Ce+Zr) × 100 (mol%)
2
X-ray diffraction angle of milled CeO powders. (Each
2
peak shifted to the higher angle with progressing milling.) Figure 31.4
The relation between ZrO content and partial OSC of
2
CeO –ZrO solid solution: (A) high-temperature reduction
2
2
at 1200 C in CO (B) mechanical alloying of CeO –ZrO 2
2
500 (C) solid-phase reaction of CeO –ZrO (CeO and
2
2
2
impregnated ZrO heated at 700 C for 5 h in air)
2
(D)
phase Ce Zr O , dotted line (E) dotted line
2
8
2
400 corresponds to theoretical value.
OSC ( molO 2 /g) 300 phase because the phase was made by the oxidation of
phase (Ce Zr O ) in early years, and the phase was
200
2
7
2
renamed as
phase Ce Zr O [25]. The
phase
2
8
2
(Ce Zr O ) has extremely large OSC. Its maximum
2
2
8
100 OSC reaches almost theoretical limit at the composi-
tion of 50 mol% of ZrO . In the composition, almost
2
all cerium ions included in the material can become
0
0 20 40 60 80 trivalent. Fig. 31.4 shows a comparison of OSC data
of (A)
phase Ce Zr O , (B) mechanically alloyed
2
8
2
ZrO 2 Content Zr/ (Ce+Zr) × 100 (mol%) CeO –ZrO 2 and (C) solid-phase reaction of
2
CeO –ZrO (zirconium salt was impregnated onto
2
2
Figure 31.3 CeO powder and then neutralized and heated at
2
The relation between ZrO content and OSC of 700 C for 5 h in air), in which OSC are shown by
2
CeO –ZrO solid solution. ( ): By mechanical alloying using the amount of O per mol of Ce (partial OSC).
2 2 2
method by using high-energy milling. ( ): By solid-phase The pyrochlore phase has an ordered arrangement of
reaction of CeO and impregnated ZrO . cation, and the ordered arrangement is kept in the
2 2
phase Ce Zr O (Fig. 31.5). The crystal structure of
8
2
2
the
phase Ce Zr O was analyzed by Kisimoto et al.
2
8
2
of ZrO , came from increasing the amount of oxygen [26]. Many analytical researches of the structure are
2
not only on the surface but also inside the CeO lat- being carried out aggressively by many researchers.
2
tice [14]. This technology was put into practical use At present, intermediate oxidation phase, Ce Zr O ,
2
2
7.5
in 1997. has been found by Sasaki et al. The new phase,
Adding some explanation about CeO –ZrO binary Ce Zr O , was named as phase [27]. These analyt-
2
2
7.5
2
2
phase, the existence of pyrochlore phase ( phase ical researches are valuable for understanding the
Ce Zr O ) has been known in Ce O –ZrO in reduc- mechanism of high OSC of the solid solutions and
2
3
2
2
7
2
tive atmosphere for many years [23]. Yao et al. found compounds in CeO –ZrO binary system, and also
2
2
metastable Ce Zr O phase in CeO –ZrO system would provide a guideline for the development of
2
2
2
2
8
[24]. The metastable Ce Zr O phase was named
next-generation OSC material.
2 2 8
552