Page 613 - Book Hosokawa Nanoparticle Technology Handbook
P. 613
39 INSTANTANEOUS NANOFOAMING METHOD FOR FABRICATION OF CLOSED-POROSITY SILICA PARTICLE APPLICATIONS
o
45 950 C
40
o
900 C
35
30
V ad (cc(STP)/g sample ) 25 850 C
o
20
o
800 C
15
o
750 C
10
o
700 C
5 o
600 C
0
0 0.2 0.4 0.6 0.8 1 500 C
o
P/P (−)
0
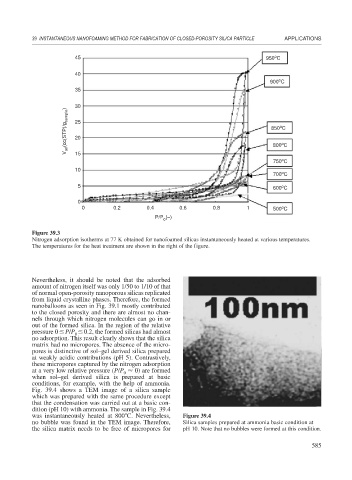
Figure 39.3
Nitrogen adsorption isotherms at 77 K obtained for nanofoamed silicas instantaneously heated at various temperatures.
The temperatures for the heat treatment are shown in the right of the figure.
Nevertheless, it should be noted that the adsorbed
amount of nitrogen itself was only 1/50 to 1/10 of that
of normal open-porosity nanoporous silicas replicated
from liquid crystalline phases. Therefore, the formed
nanoballoons as seen in Fig. 39.1 mostly contributed
to the closed porosity and there are almost no chan-
nels through which nitrogen molecules can go in or
out of the formed silica. In the region of the relative
pressure 0 P/P 0.2, the formed silicas had almost
0
no adsorption. This result clearly shows that the silica
matrix had no micropores. The absence of the micro-
pores is distinctive of sol–gel derived silica prepared
at weakly acidic contributions (pH 5). Contrastively,
these micropores captured by the nitrogen adsorption
at a very low relative pressure (P/P 0) are formed
0
when sol–gel derived silica is prepared at basic
conditions, for example, with the help of ammonia.
Fig. 39.4 shows a TEM image of a silica sample
which was prepared with the same procedure except
that the condensation was carried out at a basic con-
dition (pH 10) with ammonia. The sample in Fig. 39.4
was instantaneously heated at 800 C. Nevertheless, Figure 39.4
no bubble was found in the TEM image. Therefore, Silica samples prepared at ammonia basic condition at
the silica matrix needs to be free of micropores for pH 10. Note that no bubbles were formed at this condition.
585