Page 165 - Numerical methods for chemical engineering
P. 165
Problems 151
Table 3.2 Data for fitting quadratic two-variable model
k 1 2 3 4 5 6 7 8
θ 1 0 1 1 1 2 2 2 0
θ 2 1 0 1 2 1 2 0 2
y 1.53 1.11 2.83 4.39 4.02 5.92 2.00 3.23
2 2
r r
2 a
a
a 1 r 1 r 2 1 2 a
1
a a
r 2 2 r
2
2
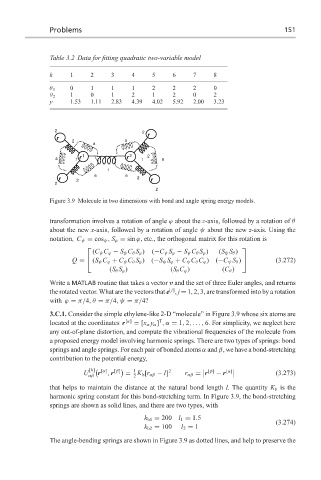
Figure 3.9 Molecule in two dimensions with bond and angle spring energy models.
transformation involves a rotation of angle ϕ about the z-axis, followed by a rotation of θ
about the new x-axis, followed by a rotation of angle ψ about the new z-axis. Using the
notation, C ψ = cos ψ , S ϕ = sin ϕ, etc., the orthogonal matrix for this rotation is
(C ψ C ϕ − S ψ C θ S ϕ ) (−C ψ S ϕ − S ψ C θ S ϕ ) (S ψ S θ )
Q = (S ψ C ϕ + C ψ C θ S ϕ )(−S ψ S ϕ + C ψ C θ C ϕ )(−C ψ S θ ) (3.272)
(S θ S ϕ ) (S θ C ϕ ) (C θ )
Write a MATLAB routine that takes a vector v and the set of three Euler angles, and returns
[j]
the rotated vector. What are the vectors that e , j = 1, 2, 3, are transformed into by a rotation
with ϕ = π/4,θ = π/4,ψ = π/4?
3.C.1. Consider the simple ethylene-like 2-D “molecule” in Figure 3.9 whose six atoms are
T
located at the coordinates r [α] = [x α y α ] ,α = 1, 2,..., 6. For simplicity, we neglect here
any out-of-plane distortion, and compute the vibrational frequencies of the molecule from
a proposed energy model involving harmonic springs. There are two types of springs: bond
springs and angle springs. For each pair of bonded atoms α and β, we have a bond-stretching
contribution to the potential energy,
[b] [α] [β] 1 2 [β]
U r ,r = K b [r αβ − l] r αβ = r − r [α] (3.273)
αβ 2
that helps to maintain the distance at the natural bond length l. The quantity K b is the
harmonic spring constant for this bond-stretching term. In Figure 3.9, the bond-stretching
springs are shown as solid lines, and there are two types, with
k b1 = 200 l 1 = 1.5
(3.274)
k b2 = 100 l 2 = 1
The angle-bending springs are shown in Figure 3.9 as dotted lines, and help to preserve the