Page 311 - Organic Electronics in Sensors and Biotechnology
P. 311
288 Chapter Seven
CH 3
CH 2 C
CO
OCH 3
UV
CH 3
C O
CH 2 C
OCH 3
+ H
or – H
CH 3 CH 2
CH 2 C
n CH 2 C
n n
CH 3 or
C CH
n 2
C O CH C
n n
OCH 3
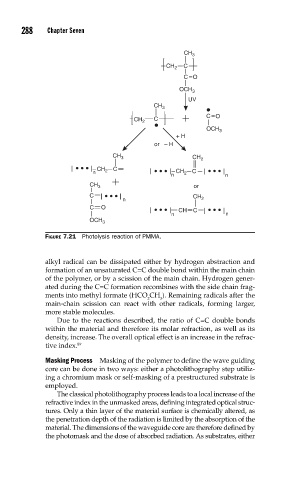
FIGURE 7.21 Photolysis reaction of PMMA.
alkyl radical can be dissipated either by hydrogen abstraction and
formation of an unsaturated C=C double bond within the main chain
of the polymer, or by a scission of the main chain. Hydrogen gener-
ated during the C=C formation recombines with the side chain frag-
ments into methyl formate (HCO CH ). Remaining radicals after the
2 3
main-chain scission can react with other radicals, forming larger,
more stable molecules.
Due to the reactions described, the ratio of C=C double bonds
within the material and therefore its molar refraction, as well as its
density, increase. The overall optical effect is an increase in the refrac-
tive index.
89
Masking Process Masking of the polymer to define the wave guiding
core can be done in two ways: either a photolithography step utiliz-
ing a chromium mask or self-masking of a prestructured substrate is
employed.
The classical photolithography process leads to a local increase of the
refractive index in the unmasked areas, defining integrated optical struc-
tures. Only a thin layer of the material surface is chemically altered, as
the penetration depth of the radiation is limited by the absorption of the
material. The dimensions of the waveguide core are therefore defined by
the photomask and the dose of absorbed radiation. As substrates, either