Page 297 - Petroleum and Gas Field Processing
P. 297
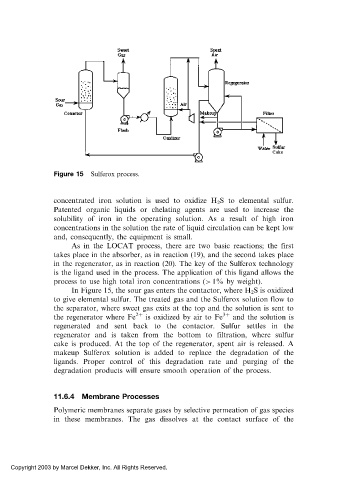
Figure 15 Sulferox process.
concentrated iron solution is used to oxidize H 2 S to elemental sulfur.
Patented organic liquids or chelating agents are used to increase the
solubility of iron in the operating solution. As a result of high iron
concentrations in the solution the rate of liquid circulation can be kept low
and, consequently, the equipment is small.
As in the LOCAT process, there are two basic reactions; the first
takes place in the absorber, as in reaction (19), and the second takes place
in the regenerator, as in reaction (20). The key of the Sulferox technology
is the ligand used in the process. The application of this ligand allows the
process to use high total iron concentrations (> 1% by weight).
In Figure 15, the sour gas enters the contactor, where H 2 S is oxidized
to give elemental sulfur. The treated gas and the Sulferox solution flow to
the separator, where sweet gas exits at the top and the solution is sent to
the regenerator where Fe 2þ is oxidized by air to Fe 3þ and the solution is
regenerated and sent back to the contactor. Sulfur settles in the
regenerator and is taken from the bottom to filtration, where sulfur
cake is produced. At the top of the regenerator, spent air is released. A
makeup Sulferox solution is added to replace the degradation of the
ligands. Proper control of this degradation rate and purging of the
degradation products will ensure smooth operation of the process.
11.6.4 Membrane Processes
Polymeric membranes separate gases by selective permeation of gas species
in these membranes. The gas dissolves at the contact surface of the
Copyright 2003 by Marcel Dekker, Inc. All Rights Reserved.