Page 295 - Petroleum and Gas Field Processing
P. 295
4NaVO 3 þ 2NaHS þ H 2 O ¼ Na 2 V 4 O 9 þ 4NaOH þ 2S ð16Þ
Na 2 V 4 O 9 þ 2NaOH þ H 2 O þ 2ADAðquinoneÞ
¼ 4NaVO 3 þ 2ADAðhydroquinoneÞ ð17Þ
1
ADAðhydroquinoneÞþ O ¼ ADAðquinoneÞ ð18Þ
2
2
The process uses anthraquinone disulfonic acid (ADA) as the organic
oxygen carrier. One of the products is finely divided sulfur and the process
is capable of treating natural gas of very low H 2 S concentrations. In the
oxidizer or regenerator, the reduced anthraquinone disulfonic acid is
reoxidized by blowing air, as shown by reaction (18). The precipitated
sulfur is overflown as froth.
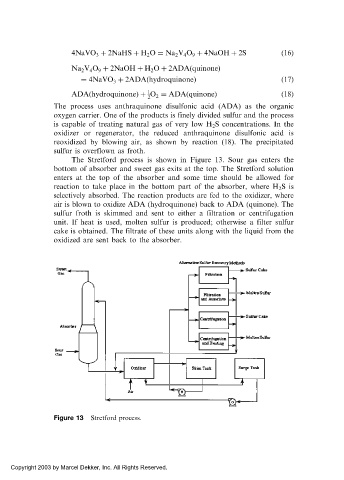
The Stretford process is shown in Figure 13. Sour gas enters the
bottom of absorber and sweet gas exits at the top. The Stretford solution
enters at the top of the absorber and some time should be allowed for
reaction to take place in the bottom part of the absorber, where H 2 Sis
selectively absorbed. The reaction products are fed to the oxidizer, where
air is blown to oxidize ADA (hydroquinone) back to ADA (quinone). The
sulfur froth is skimmed and sent to either a filtration or centrifugation
unit. If heat is used, molten sulfur is produced; otherwise a filter sulfur
cake is obtained. The filtrate of these units along with the liquid from the
oxidized are sent back to the absorber.
Figure 13 Stretford process.
Copyright 2003 by Marcel Dekker, Inc. All Rights Reserved.