Page 296 - Petroleum and Gas Field Processing
P. 296
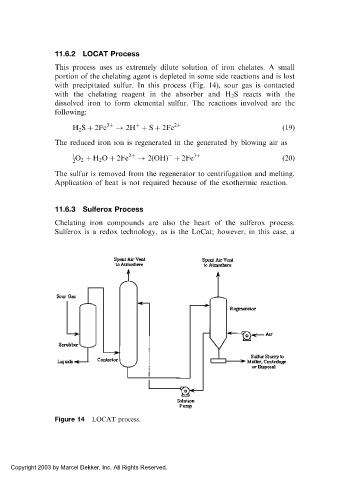
11.6.2 LOCAT Process
This process uses as extremely dilute solution of iron chelates. A small
portion of the chelating agent is depleted in some side reactions and is lost
with precipitated sulfur. In this process (Fig. 14), sour gas is contacted
with the chelating reagent in the absorber and H 2 S reacts with the
dissolved iron to form elemental sulfur. The reactions involved are the
following:
þ
H 2 S þ 2Fe 3þ ! 2H þ S þ 2Fe 2þ ð19Þ
The reduced iron ion is regenerated in the generated by blowing air as
1 2þ 3þ
2 O 2 þ H 2 O þ 2Fe ! 2ðOHÞ þ 2Fe ð20Þ
The sulfur is removed from the regenerator to centrifugation and melting.
Application of heat is not required because of the exothermic reaction.
11.6.3 Sulferox Process
Chelating iron compounds are also the heart of the sulferox process.
Sulferox is a redox technology, as is the LoCat; however, in this case, a
Figure 14 LOCAT process.
Copyright 2003 by Marcel Dekker, Inc. All Rights Reserved.