Page 114 - Photoreactive Organic Thin Films
P. 114
3. PHOTO-ORIENTATION BY PHOTOISOMERIZATION 93
The quantum yields are reasonably small for photoisomerization
processes in polymeric environments, meaning that molecular movement can
be hindered far below the polymer Tg. These quantum yields are in
38
B
agreement with those reported in the literature. P 2(cos ^33) P£MS~* MS =
0.493 shows that the orientation of the chromophore is partially retained,
i.e., not thermalized, after the UV-light-induced A—»B photoisomerization.
^33 = 71.25 degrees demonstrates that the direction of the 365 and 633 nm
transitions of the B isomer are nearly perpendicular to each other, a feature
that explains the observed inversion of the sign of the anisotropy of photo-
oriented SP in PMMA for the UV versus the visible transition band. Next, I
discuss the photo-orientation features of DE in PMMA.
Diarylethenes contrast with spiropyrans by the thermal stability of the B
isomer, a feature that brings about interesting photo-orientation effects in
spectrally distinguishable photoisomers. The order parameter is independent
of the irradiation light intensity at the photostationary state. Quantified
photo-orientation of diarylethenes reveals that the closed form of such
chromophores also exhibits perpendicular UV and_yisible transition dipole
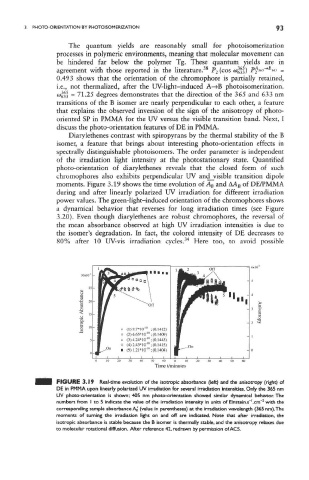
moments. Figure 3.19 shows the time evolution of A B and AA B of DE/PMMA
during and after linearly polarized UV irradiation for different irradiation
power values. The green-light-induced orientation of the chromophores shows
a dynamical behavior that reverses for long irradiation times (see Figure
3.20). Even though diarylethenes are robust chromophores, the reversal of
the mean absorbance observed at high UV irradiation intensities is due to
the isomer's degradation. In fact, the colored intensity of DE decreases to
34
80% after 10 UV-vis irradiation cycles. Here too, to avoid possible
FIGURE 3.19 Real-time evolution of the isotropic absorbance (left) and the anisotropy (right) of
DE in PMMA upon linearly polarized UV irradiation for several irradiation intensities. Only the 365 nm
UV photo-orientation is shown; 405 nm photo-orientation showed similar dynamical behavior. The
l
2
numbers from I to 5 indicate the value of the irradiation intensity in units of Einstein.s~,cmf with the
corresponding sample absorbance AJ (value in parentheses) at the irradiation wavelength (365 nm),The
moments of turning the irradiation light on and off are indicated. Note that after irradiation, the
isotropic absorbance is stable because the B isomer is thermally stable, and the anisotropy relaxes due
to molecular rotational diffusion. After reference 42, redrawn by permission of ACS.