Page 117 - Photoreactive Organic Thin Films
P. 117
ZOUHEIR SEKKAT
A<h-B isomerization and that the observed anisotropy at both 532 and
365 nm is due to the orientation of isomer B only. If orientation occurs in A
at any time by green irradiation, some anisotropy should remain at 365 nm
after all B are isomerized to A. This behavior is theoretically rationalized by
B
p^365~* 532 _ o, because the orientation of A is proportional to that of B
365 532
through P^ "^ (vide infra).
The lack of orientation in A may be due to the large amount of energy
that needs to be dissipated during the photochemical process induced by the
532 or 546 nm photon; perhaps when the molecule is excited, it shakes
strongly before it relaxes, wjff = 74.3 and 6/532 = 61.3 degrees demonstrate
that the direction of the UV, i.e., 365 and 405 nm, and visible, i.e., 532 nm,
transitions of the B isomer {the closed form) are oriented toward perpen-
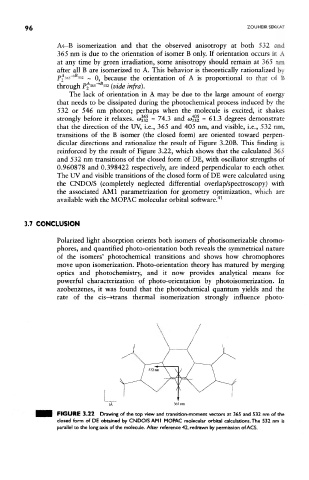
dicular directions and rationalize the result of Figure 3.20B. This finding is
reinforced by the result of Figure 3.22, which shows that the calculated 365
and 532 nm transitions of the closed form of DE, with oscillator strengths of
0.960878 and 0.398422 respectively, are indeed perpendicular to each other.
The UV and visible transitions of the closed form of DE were calculated using
the CNDO/S (completely neglected differential overlap/spectroscopy) with
the associated AMI parametrization for geometry optimization, which are
available with the MOPAC molecular orbital software. 41
3.7 CONCLUSION
Polarized light absorption orients both isomers of photisomerizable chromo-
phores, and quantified photo-orientation both reveals the symmetrical nature
of the isomers' photochemical transitions and shows how chromophores
move upon isomerization. Photo-orientation theory has matured by merging
optics and photochemistry, and it now provides analytical means for
powerful characterization of photo-orientation by photoisomerization. In
azobenzenes, it was found that the photochemical quantum yields and the
rate of the cis—»trans thermal isomerization strongly influence photo-
iA
FIGURE 3.22 Drawing of the top view and transition-moment vectors at 365 and 532 nm of the
closed form of DE obtained by CNDO/S AM I MOB^C molecular orbital calculations. The 532 nm is
parallel to the long axis of the molecule. After reference 42, redrawn by permission of ACS.