Page 116 - Photoreactive Organic Thin Films
P. 116
3. PHOTO-ORIENTATION BY PHOTOISOMERIZATIOIM 95
X'*10
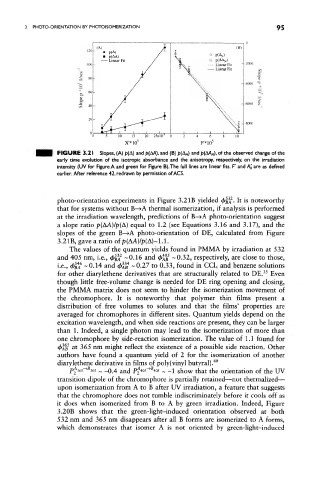
FIGURE 3.21 Slopes, (A) p(A) and p(AA), and (B) p(A N ) and f>(M N), of the observed change of the
early time evolution of the isotropic absorbance and the anisotropy, respectively, on the irradiation
intensity (UV for Figure A and green for Figure B).The full lines are linear fits. F' and AQ are as defined
earlier. After reference 42, redrawn by permission of ACS.
2
photo-orientation experiments in Figure 3.2 IB yielded <j&|i . It is noteworthy
that for systems without B— »A thermal isomerization, if analysis is performed
at the irradiation wavelength, predictions of B-»A photo-orientation suggest
a slope ratio p(AA)/p(A) equal to 1.2 (see Equations 3.16 and 3.17), and the
slopes of the green B— »A photo-orientation of DE, calculated from Figure
3.21B, gave a ratio of p(AA)//?(A)~l.l.
The values of the quantum yields found in PMMA by irradiation at 532
5
2
and 405 nm, i.e., ^>|^ -0.16 and ^° B -0.32, respectively, are close to those,
i.e., <f>BA -0.14 and <$$ -0.27 to 0.33, found in CC1 4 and benzene solutions
35
for other diarylethene derivatives that are structurally related to DE. Even
though little free-volume change is needed for DE ring opening and closing,
the PMMA matrix does not seem to hinder the isomerization movement of
the chromophore. It is noteworthy that polymer thin films present a
distribution of free volumes to solutes and that the films' properties are
averaged for chromophores in different sites. Quantum yields depend on the
excitation wavelength, and when side reactions are present, they can be larger
than 1. Indeed, a single photon may lead to the isomerization of more than
one chromophore by side-reaction isomerization. The value of 1.1 found for
5
t^! at 365 nm might reflect the existence of a possible side reaction. Other
authors have found a quantum yield of 2 for the isomerization of another
diarylethene derivative in films of poly( vinyl butyral). 40
>B 405 405
p^?65- 365 ^ _o.4 and P^ ^ - -1 show that the orientation of the UV
transition dipole of the chromophore is partially retained — not thermalized —
upon isomerization from A to B after UV irradiation, a feature that suggests
that the chromophore does not tumble indiscriminately before it cools off as
it does when isomerized from B to A by green irradiation. Indeed, Figure
3.20B shows that the green-light-induced orientation observed at both
532 nm and 365 nm disappears after all B forms are isomerized to A forms,
which demonstrates that isomer A is not oriented by green-light-induced